2. 730000 兰州,兰州大学:第一医院麻醉科
2. Department of Anesthesiology, the First Hospital of Lanzhou University, Lanzhou, Gansu Province, 730000, China
脓毒症是宿主对感染的反应异常导致危及生命的器官功能障碍[1]。急性肾损伤(acute kidney injury, AKI)是脓毒症的一种常见的并发症,也是进展为慢性肾脏疾病的一个危险因素[2]。脓毒症AKI的全球发病率约为每年1 100万例[3]。高达60%的脓毒症患者在ICU中发现脓毒症AKI[4],而且临床上被诊断为脓毒症AKI的患者往往存在更高的死亡风险[5]。
炎症反应是宿主抵抗感染的主要防御机制,但炎症失衡可导致组织和器官的进一步损伤,这也是脓毒症主要的发病机制[6]。最新研究表明,有氧糖酵解在宿主防御感染的免疫反应中发挥核心作用,是炎症性疾病的一个新靶点[7]。脓毒症时,小鼠肾脏组织代谢可转变为有氧糖酵解,使糖酵解水平和糖酵解途径多种关键酶水平增加,抑制有氧糖酵解可以减轻脓毒症AKI,提高脓毒症小鼠存活率[8]。但其具体机制目前报道较少,需要进一步研究来明确。
越来越多的证据表明PI3K-AKT-mTOR信号通路激活参与调控细胞有氧糖酵解[9-10],HU等[11]发现LPS可通过激活肺成纤维细胞PI3K-AKT-mTOR信号通路,上调6-磷酸果糖激酶-2/果糖-2, 6-二磷酸酶3 (6-phosphofructo-2-kinase/fructose-2, 6-biphosphatase 3,PFKFB3),加速有氧糖酵解,促进肺成纤维细胞的胶原合成。
WIN55212-2 (WIN)是一种具有抗炎特性的大麻素受体激动剂,研究表明WIN可减轻LPS诱导的人内皮细胞的炎症激活[12]。尚有研究发现WIN可通过miR-29b-3p/FOXO3/PFKFB3信号轴抑制糖酵解,减轻LPS诱导的急性肺损伤(acute lung injury,ALI)[13]。而WIN能否通过抑制有氧糖酵解减轻脓毒症AKI及其与AKT/mTOR/PFKFB3信号通路之间的关系,尚有待探明。因此,本研究采用腹腔注射LPS制备小鼠脓毒症AKI模型,探讨WIN是否可以通过调控AKT/mTOR/PFKFB3信号通路活性,抑制有氧糖酵解,减轻脓毒症小鼠AKI。
1 材料与方法 1.1 实验动物与主要试剂SPF级别6~8周雄性健康C57BL/6小鼠,体质量20~25 g,购自兰州大学实验动物中心[动物许可证号:SCXK(甘)2018-0002]。小鼠正常进食,人工光照和黑暗时间12 h交替,适应性饲养1周后开始实验。LPS购自美国Sigma公司;mTOR激活剂MHY1485购自美国MCE公司;WIN55212-2购自Cayman公司;抗p-AKT、抗p-mTOR抗体均购于博奥森公司;抗PFKFB3抗体购于Abcam公司;IL-18、IL-1β、Scr、KIM-1、LDHA、乳酸酶联免疫吸附(ELISA)测定试剂盒均购自上海酶联生物科技有限公司。实验过程中动物处理措施符合伦理学要求,已获兰州大学第一医院伦理委员会批准(LDYYLL2023-320)。
1.2 实验分组与处理24只小鼠按照随机数字表法分为4组(n=6):①对照组(Control组);②脓毒症组(LPS组),腹腔注射LPS(10 mg/kg)[14];③脓毒症+WIN55212-2组(LPS+ WIN组),LPS处理前30 min腹腔注射WIN 1 mg/kg[13];④脓毒症+WIN55212-2+MHY组(LPS+WIN+MHY组),LPS处理前1 d腹腔注射MHY1485 10 mg/kg[15],LPS处理前30 min腹腔注射MHY1485 10 mg/kg和WIN 1 mg/kg。Control组和LPS组于上述相同时间腹腔注射等量二甲基亚砜。
1.3 标本采集造模后24 h,戊巴比妥钠50 mg/kg腹腔注射麻醉小鼠后,将其仰卧于固定板上;剪去心前区毛发并消毒,以食指指腹定位小鼠心脏搏动区; 手持1 mL注射器穿刺心搏最强处,收集足量血液标本。静置30 min后以3 000 r/min,离心15 min,取上清液于-80 ℃冰箱保存。心脏取血后安乐死小鼠。常规剪去毛发、消毒,暴露腹腔; 取小鼠双侧肾脏组织,PBS冲洗; 将一侧肾脏组织固定于4%多聚甲醛溶液(用于病理学观察);将另一侧肾脏组织于-80 ℃冰箱储存(用于后续实验)。
1.4 HE染色取肾脏组织后,置于4%多聚甲醛中固定48 h,脱水后石蜡包埋、切片、HE染色,显微镜下观察肾脏组织病理结构改变。采用Paller氏评分[16]评估肾小管损伤程度,即在200倍高倍镜下取10个高倍镜视野合计100个肾小管。肾小管明显扩张、细胞扁平(1分);肾小管内出现管型(2分);肾小管管腔内有脱落、坏死细胞,但未成管型或细胞碎片(1分);上皮细胞颗粒变性(1分);空泡变性(1分);细胞核固缩(1分)。
1.5 ELISA检测肾脏组织称质量,按照1 g组织加入5 mL PBS的比例加入PBS,在冰水浴中尽量剪碎组织,随后按照每20 mg肾脏组织中加入150~200 μL的RIPA裂解液的比例加入裂解液,在冰上使用匀浆机制备成10%肺组织匀浆。将制备好的10%匀浆使用离心机4 ℃,3 000×g离心10 min,离心后的匀浆取上清液进行分装,-80 ℃放置保存。按照ELISA试剂盒说明书检测肾脏组织匀浆炎症因子IL-1β、IL-18水平以及LDHA的含量。
取出-80 ℃储存的血清,逐级解冻后作为待测样品。按照ELISA试剂盒说明书进行检测。使用酶标仪测定各孔光密度值[D(450)]。Excel绘制标准曲线,计算各样本血清Scr、KIM-1和乳酸的含量。
1.6 Western blot检测取-80 ℃肾脏组织匀浆,蛋白定量,经十二烷基硫酸钠-聚丙烯酰胺凝胶电泳2 h,电转移至聚偏二氟乙烯(PVDF) 膜上,洗膜后加入p-AKT、p-mTOR、PFKFB3一抗和β-actin内参蛋白4 ℃孵育过夜。次日洗膜,加入辣根过氧化物酶(HRP)标记的山羊抗兔二抗(稀释度均为1∶100 000)37 ℃摇床孵育2 h,含吐温-20的Tris-HCl缓冲盐溶液(TBST) 充分洗膜。电化学发光法显色曝光,采集图像,用Image J软件分析各组条带灰度值,以目标蛋白与内参蛋白的灰度值比值反映目标蛋白相对表达量。
1.7 统计学分析采用Graphpad Prism 8.0软件进行数据分析。实验数据均为计量资料且符合正态分布,以x±s表示,多组间比较采用单因素方差分析,2组间比较采用LSD-t检验。P<0.05为差异有统计学意义。
2 结果 2.1 小鼠一般状态LPS造模后24 h,Control组小鼠精神状态、饮食、活动、呼吸频率及对刺激的反应较造模之前无明显改变;LPS组小鼠出现发热,心率、呼吸频率明显加快,口鼻腔分泌物增多,精神萎靡,嗜睡,蜷缩,竖毛,少动,拒食或少食,眼角出现分泌物;LPS+WIN组上述表现较LPS组轻微,LPS+WIN+MHY组上述表现较LPS+WIN组明显加重。
2.2 各组小鼠肾脏组织病理结构改变光镜下Control组为正常肾脏组织,肾脏结构完整,无出血和炎症浸润;LPS组肾小管上皮细胞水肿,细胞体积变大,肾小球结构紊乱,肾小管管腔变窄,间质内有明显出血及大量炎症细胞浸润;LPS+WIN组病理改变明显减轻,肾脏结构较完整,间质仅有少量出血及炎症细胞浸润;LPS+WIN+MHY组病理改变较LPS+WIN组加重,肾脏组织结构破坏,间质内出血及炎症细胞浸润增多,见图 1。
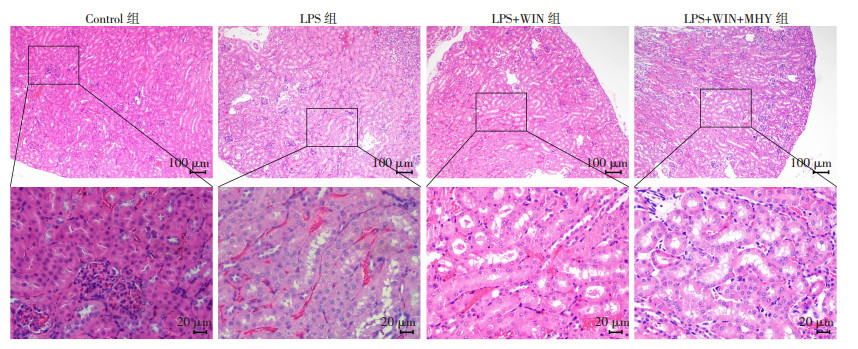
|
| 图 1 HE染色观察各组小鼠肾脏组织病理变化 |
与Control组相比,LPS组Paller评分明显增高(P<0.05);与LPS组相比,LPS+WIN组在WIN预处理后Paller评分明显下降(P<0.05);相较于LPS+WIN组,LPS+WIN+MHY组Paller评分升高,差异均有统计学意义(P<0.05),见图 2。
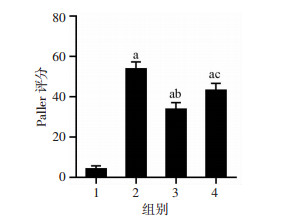
|
| 1:Control组;2:LPS组;3:LPS+WIN组;4:LPS+WIN+MHY组;a:P<0.05,与Control组比较;b:P<0.05,与LPS组比较;c:P<0.05,与LPS+WIN组比较 图 2 各组小鼠肾脏Paller评分(n=6,x±s) |
2.3 各组小鼠肾脏组织IL-1β、IL-18和糖酵解关键酶LDHA含量
ELISA检测结果显示:与Control组相比,LPS组中IL-1β、IL-18、LDHA含量显著升高(P<0.05);与LPS组相比,LPS+WIN组在WIN预处理后IL-1β、IL-18、LDHA含量相对下降(P<0.05);与LPS+WIN组相比,LPS+WIN+MHY组IL-1β、IL-18、LDHA含量增高,差异均有统计学意义(P<0.05),见图 3。
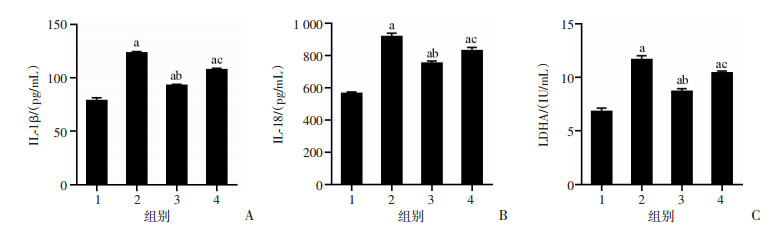
|
| 1:Control组;2:LPS组;3:LPS+WIN组;4:LPS+WIN+MHY组;a:P<0.05,与Control组比较;b:P<0.05,与LPS组比较;c:P<0.05,与LPS+WIN组比较 图 3 ELISA法检测各组小鼠肾脏组织IL-1β(A)、IL-18(B)和LDHA(C)的含量(n=6,x±s) |
2.4 各组小鼠血清肾损伤标志物Scr、KIM-1和糖酵解产物乳酸含量
ELISA检测结果显示:与Control组相比,LPS组Scr、KIM-1、乳酸含量明显增加(P<0.05);与LPS组相比,LPS+WIN组Scr、KIM-1、乳酸含量相对下降(P<0.05);与LPS+WIN组相比,LPS+WIN+MHY组Scr、KIM-1、乳酸含量增高,差异均有统计学意义(P<0.05),见图 4。
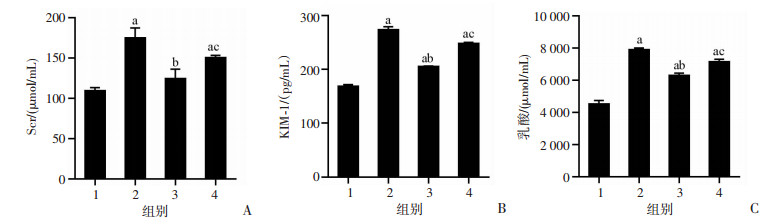
|
| 1:Control组;2:LPS组;3:LPS+WIN组;4:LPS+WIN+MHY组;a:P<0.05,与Control组比较;b:P<0.05,与LPS组比较;c:P<0.05,与LPS+WIN组比较 图 4 ELISA法检测各组小鼠血清Scr(A)、KIM-1(B)和乳酸(C)的含量(n=6,x±s) |
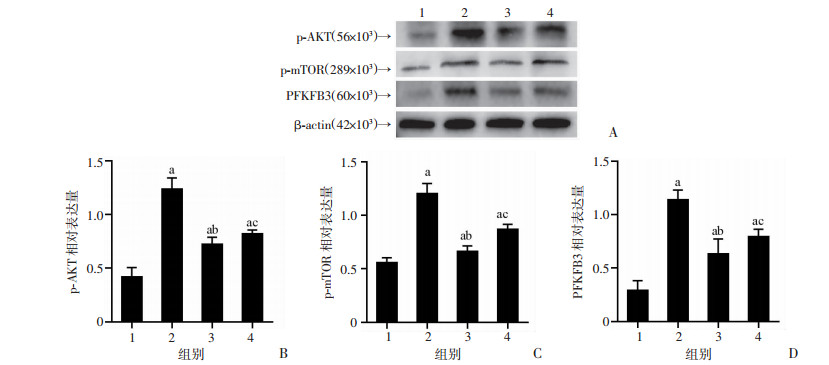
2.5 各组小鼠肾脏组织p-AKT、p-mTOR及PFKFB3蛋白表达水平
Western blot检测结果显示:与Control组相比,LPS组p-AKT、p-mTOR及PFKFB3表达显著升高(P<0.05);与LPS组相比,LPS+WIN组p-AKT、p-mTOR及PFKFB3表达相对下降(P<0.05);相较于LPS+WIN组,LPS+WIN+MHY组p-AKT、p-mTOR及PFKFB3表达增高,差异均有统计学意义(P<0.05),见图 5。
|
|
1:Control组;2:LPS组;3:LPS+WIN组;4:LPS+WIN+MHY组;a:P<0.05,与Control组比较;b:P<0.05,与LPS组比较;c:P<0.05,与LPS+WIN组比较 A:Western blot检测肾脏组织p-AKT、p-mTOR及PFKFB3蛋白表达水平;B:p-AKT蛋白相对表达量;C:p-mTOR蛋白相对表达量;D:PFKFB3蛋白相对表达量 图 5 Western blot检测各组小鼠肾脏组织p-AKT、p-mTOR及PFKFB3蛋白表达水平(n=6,x±s) |
3 讨论
本研究采用腹腔注射LPS的方法制备脓毒症小鼠模型。LPS组小鼠造模后24 h,出现发热,心率、呼吸频率明显加快,口鼻腔分泌物增多,精神萎靡,嗜睡,蜷缩,竖毛,少动,拒食或少食等症状,小鼠肾功能明显下降,肾脏病理损伤加重,炎症因子IL-18、IL-1β水平显著升高,证实LPS脓毒症模型成功。
WIN作为大麻素受体1和2的强效激动剂,在细胞和动物模型中发挥广泛的抗炎作用[17]。本研究发现,LPS+WIN组肾脏结构破坏,间质内出血及炎症因子浸润等病理改变减轻,炎症因子IL-1β、IL-18含量降低,血清肾损伤标志物Scr、KIM-1的含量也明显下降,说明WIN可减轻脓毒症AKI中的炎症反应。PÉREZ-DIEGO等[18]研究也证实,WIN可降低脓毒症小鼠血清TNF-α、IL-1β、IL-6等炎症因子水平;FIELDS等[19]研究同样表明,在人类星形胶质细胞中,WIN预先给药可以显著抑制IL-1β诱导的炎症反应。
IL-18作为IL-1超家族的一员,是急性肾损伤的重要介质,可在一定程度上反映肾损伤的水平[20]。KIM-1在急性肾损伤后早期释放,已被广泛用作AKI早期诊断标志物[21]。因此本实验选择肾组织IL-18以及血清KIM-1、Scr用以评估脓毒症AKI小鼠肾功能。
在脓毒症期间,细胞能量代谢从氧化磷酸化转变为糖酵解,这种现象被称为代谢重编程[22]。脓毒症期间有氧糖酵解可以激活炎症小体,释放IL-1β、IL-18和高迁移率族蛋白B1(high mobility group box 1 protein,HMGB1)等促炎因子,而限制糖酵解途径可降低脓毒症早期炎症因子的释放,减轻炎症反应[23-24]。LDHA作为糖酵解途径最后一步的关键酶,可催化丙酮酸生成乳酸,而负责乳酸的形成使LDHA成为有氧糖酵解的关键参与者[25]。果糖-2,6-二磷酸(fructose-2, 6-bisphosphate, F-2, 6-BP)是糖酵解限速酶磷酸果糖激酶-1(phosphofructokinase- 1, PFK-1)的强效变构激活剂,PFKFB3可通过调节F-2, 6-BP合成从而在有氧糖酵解中起关键作用[26]。本实验观察到,LPS组LDHA和PFKFB3表达上调,糖酵解终产物血清乳酸生成增加,且伴随着IL-1β、IL-18水平升高,肾脏病理损伤加重,而WIN预处理糖酵解水平下降,炎症因子含量降低,血清Scr、KIM-1也随之下降,肾损伤有所减轻,表明WIN可通过下调糖酵解水平,抑制炎症反应,减轻脓毒症AKI。LUO等[27]研究表明,靶向抑制糖酵解,可降低IL-1β等炎症因子的激活与释放,减轻脓毒症小鼠肝、肾损伤,提高小鼠存活率,这与本实验结果相一致。HE等[13]经体内、体外实验证实,WIN预处理抑制糖酵解,降低肺泡巨噬细胞向促炎的M1表型极化,减少炎症因子释放,减轻脓毒症ALI。
为了阐明其保护机制,本研究在WIN基础上使用mTOR激活剂MHY1485干预小鼠,MHY1485可通过靶向mTOR的ATP结构域,调节该通路活性[28]。结果发现,MHY1485可拮抗WIN对有氧糖酵解的抑制作用,肾脏糖酵解水平重新升高,炎症因子含量升高,肾脏病理损伤加重,p-AKT、p-mTOR及PFKFB3的表达较LPS+WIN组明显升高。因此我们推测WIN可能通过抑制AKT/mTOR/PFKFB3信号通路激活,下调糖酵解水平,减轻肾脏组织炎症反应。既往研究表明AKT-mTOR信号通路可通过调控下游效应分子,调节细胞生长、增殖和有氧糖酵解[29]。AKT的激活可以动员葡萄糖转运蛋白(glucose transporter, GLUT),激活己糖激酶2(hexokinase2, HK2),从而增强有氧糖酵解和促进癌细胞生长[30]。mTOR可上调PFKFB3的表达加速糖酵解速率[31]。这些研究结果与本实验高度一致。最新研究也发现,WIN能够抑制LPS诱导的AKT和p70S6K(mTOR的主要下游作用靶点)的磷酸化,表明WIN可以抑制LPS诱导的人单核细胞来源的树突状细胞mTOR的活化,从而通过激活自噬抑制代谢重编程,促进调节性T细胞的产生,保护小鼠抵抗LPS诱导的脓毒症[32]。
本研究也存在一定局限性,实验仅在动物模型观察WIN在脓毒症AKI中的作用,未在细胞水平进一步验证。WIN也可能通过其他信号通路发挥脓毒症保护作用,还需进一步研究。
综上所述,WIN55212-2可以减轻脓毒症AKI,其机制可能是通过抑制AKT/mTOR/PFKFB3信号通路,降低肾脏糖酵解水平,减轻炎症反应。
| [1] |
FERNANDO S M, ROCHWERG B, SEELY A J E. Clinical implications of the third international consensus definitions for sepsis and septic shock (Sepsis-3)[J]. CMAJ, 2018, 190(36): E1058-E1059. |
| [2] |
KUWABARA S, GOGGINS E, OKUSA M D. The pathophysiology of sepsis-associated AKI[J]. Clin J Am Soc Nephrol, 2022, 17(7): 1050-1069. |
| [3] |
MANRIQUE-CABALLERO C L, DEL RIO-PERTUZ G, GOMEZ H. Sepsis-associated acute kidney injury[J]. Crit Care Clin, 2021, 37(2): 279-301. |
| [4] |
POSTON J T, KOYNER J L. Sepsis associated acute kidney injury[J]. BMJ, 2019, k4891. |
| [5] |
KALANTARI K, ROSNER M H. Recent advances in the pharmacological management of sepsis-associated acute kidney injury[J]. Expert Rev Clin Pharmacol, 2021, 14(11): 1401-1411. |
| [6] |
SINGBARTL K, FORMECK C L, KELLUM J A. Kidney-immune system crosstalk in AKI[J]. Semin Nephrol, 2019, 39(1): 96-106. |
| [7] |
SUN L Z, YANG X F, YUAN Z Y, et al. Metabolic reprogramming in immune response and tissue inflammation[J]. Arterioscler Thromb Vasc Biol, 2020, 40(9): 1990-2001. |
| [8] |
TAN C Y, GU J, LI T, et al. Inhibition of aerobic glycolysis alleviates sepsis-induced acute kidney injury by promoting lactate/Sirtuin 3/AMPK-regulated autophagy[J]. Int J Mol Med, 2021, 47(3): 19. |
| [9] |
LIAO S, LIANG L, YUE C X, et al. CD38 is involved in cell energy metabolism via activating the PI3K/AKT/mTOR signaling pathway in cervical cancer cells[J]. Int J Oncol, 2020, 57(1): 338-354. |
| [10] |
ZHANG T, ZHANG Y G, YANG Z H, et al. Echinococcus multilocularis protoscoleces enhance glycolysis to promote M2 macrophages through PI3K/Akt/mTOR signaling pathway[J]. Pathog Glob Health, 2023, 117(4): 409-416. |
| [11] |
HU X T, XU Q Y, WAN H X, et al. PI3K-Akt-mTOR/PFKFB3 pathway mediated lung fibroblast aerobic glycolysis and collagen synthesis in lipopolysaccharide-induced pulmonary fibrosis[J]. Lab Invest, 2020, 100(6): 801-811. |
| [12] |
WILHELMSEN K, KHAKPOUR S, TRAN A, et al. The endocannabinoid/endovanilloid N-arachidonoyl dopamine (NADA) and synthetic cannabinoid WIN55, 212-2 abate the inflammatory activation of human endothelial cells[J]. J Biol Chem, 2014, 289(19): 13079-13100. |
| [13] |
HE Q A, YIN J, ZOU B S, et al. WIN55212-2 alleviates acute lung injury by inhibiting macrophage glycolysis through the miR-29b-3p/FOXO3/PFKFB3 axis[J]. Mol Immunol, 2022, 149: 119-128. |
| [14] |
LIU L Q, YAN M J, YANGR, et al. Adiponectin attenuates lipopolysaccharide-induced apoptosis by regulating the Cx43/PI3K/AKT pathway[J]. Front Pharmacol, 2021, 12: 644225. |
| [15] |
ZHOU J L, YAO W, LI C Y, et al. Administration of follicle-stimulating hormone induces autophagy via upregulation of HIF-1α in mouse granulosa cells[J]. Cell Death Dis, 2017, 8(8): e3001. |
| [16] |
PALLER M S, HOIDAL J R, FERRIS T F. Oxygen free radicals in ischemic acute renal failure in the rat[J]. J Clin Invest, 1984, 74(4): 1156-1164. |
| [17] |
SU S H, WU Y F, LIN Q, et al. Cannabinoid receptor agonist WIN55, 212-2 and fatty acid amide hydrolase inhibitor URB597 ameliorate neuroinflammatory responses in chronic cerebral hypoperfusion model by blocking NF-κB pathways[J]. Naunyn Schmiedebergs Arch Pharmacol, 2017, 390(12): 1189-1200. |
| [18] |
PÉREZ-DIEGO M, ANGELINA A, MARTÍN-CRUZ L, et al. Cannabinoid WIN55, 212-2 reprograms monocytes and macrophages to inhibit LPS-induced inflammation[J]. Front Immunol, 2023, 14: 1147520. |
| [19] |
FIELDS J A, SWINTON M K, MONTILLA-PEREZ P, et al. The cannabinoid receptor agonist, WIN-55212-2, suppresses the activation of proinflammatory genes induced by interleukin 1 beta in human astrocytes[J]. Cannabis Cannabinoid Res, 2022, 7(1): 78-92. |
| [20] |
MOLEDINA D G, PARIKH C R. Phenotyping of acute kidney injury: beyond serum creatinine[J]. Semin Nephrol, 2018, 38(1): 3-11. |
| [21] |
DIAO H Y, ZHU W, LIU J, et al. Salvianolic acid A improves rat kidney injury by regulating MAPKs and TGF-β1/smads signaling pathways[J]. Molecules, 2023, 28(8): 3630. |
| [22] |
ZHENG Z B, MA H, ZHANG X, et al. Enhanced glycolytic metabolism contributes to cardiac dysfunction in polymicrobial Sepsis[J]. J Infect Dis, 2017, 215(9): 1396-1406. |
| [23] |
LIU W Z, LIU T Y, ZHENG Y J, et al. Metabolic reprogramming and its regulatory mechanism in sepsis-mediated inflammation[J]. J Inflamm Res, 2023, 16: 1195-1207. |
| [24] |
WANG L, WANG D G, ZHANG T L, et al. The role of immunometabolism in macrophage polarization and its impact on acute lung injury/acute respiratory distress syndrome[J]. Front Immunol, 2023, 14: 1117548. |
| [25] |
POUYSSÉGUR J, MARCHIQ I, PARKS S K, et al. 'Warburg effect' controls tumor growth, bacterial, viral infections and immunity—Genetic deconstruction and therapeutic perspectives[J]. Semin Cancer Biol, 2022, 86(Pt 2): 334-346. |
| [26] |
BAO Y F, ZHOU L, DAI D Q, et al. Discover potential inhibitors for PFKFB3 using 3D-QSAR, virtual screening, molecular docking and molecular dynamics simulation[J]. J Recept Signal Transduct, 2018, 38(5/6): 413-431. |
| [27] |
LUO P, ZHANG Q, ZHONG T Y, et al. Celastrol mitigates inflammation in sepsis by inhibiting the PKM2-dependent Warburg effect[J]. Mil Med Res, 2022, 9(1): 22. |
| [28] |
CAI Y, GAO Q, MENG J H, et al. Puerarin suppresses glycolysis and increases cisplatin chemosensitivity in oral squamous cell carcinoma via FBXW7/mTOR signaling[J]. Nutr Cancer, 2023, 75(3): 1028-1037. |
| [29] |
CHOU W C, RAMPANELLI E, LI X, et al. Impact of intracellular innate immune receptors on immunometabolism[J]. Cell Mol Immunol, 2022, 19(3): 337-351. |
| [30] |
ABDEL-WAHAB A F, MAHMOUD W, AL-HARIZY R M. Targeting glucose metabolism to suppress cancer progression: prospective of anti-glycolytic cancer therapy[J]. Pharmacol Res, 2019, 150: 104511. |
| [31] |
FENG Y H, WU L S. mTOR up-regulation of PFKFB3 is essential for acute myeloid leukemia cell survival[J]. Biochem Biophys Res Commun, 2017, 483(2): 897-903. |
| [32] |
ANGELINA A, PÉREZ-DIEGO M, LÓPEZ-ABENTE J, et al. Cannabinoids induce functional Tregs by promoting tolerogenic DCs via autophagy and metabolic reprograming[J]. Mucosal Immunol, 2022, 15(1): 96-108. |




