急性肺损伤(acute lung injury,ALI)是一种由多种诱因引起的急性肺炎症性疾病,重症可发展成急性呼吸窘迫综合征(acute respiratory sistress syndrome,ARDS)而致死[1]。其临床症状发展迅速,可致多器官功能障碍。目前临床上主要有控制感染、机械通气、抗氧化治疗、糖皮质激素治疗等几种治疗方法。但以上治疗方法存在疗效不确切和副作用大等问题[2],因此需要寻找新型的有效治疗ALI的药物。
急性肺损伤的病因较为复杂,炎症级联反应、中性粒细胞的粘附及肺水肿与其发生发展密切相关[3]。炎症反应在ALI的病理过程中起着重要作用,其可通过信号转导途径激活炎症细胞,释放大量炎性细胞因子,如TNF-α、IL-1β及IL-6。脂多糖又名内毒素(lipopolysaccharide,LPS),它是革兰阴性菌外膜的重要组成成分,常用于构建急性肺损伤模型,是包括急性肺损伤在内的各种炎症性疾病最常见的致病因素之一[4-5]。
老鹳草素(geraniin)是睡莲花、老鹳草、叶下珠等植物的主要活性成分,属于β-D-吡喃葡萄糖,是一种黄色的结晶状的多酚类物质,它的化学式为C41H28O27,相对分子质量为952.64,易溶于水、甲醇、乙醇等极性大的溶剂[6]。其具有多种生物活性如抗炎、抗肿瘤、抗氧化、抗病毒、抗菌等[7-8]。然而其对LPS诱导的ALI小鼠的作用报道较少。因此,本研究采用LPS诱导小鼠ALI模型,探讨老鹳草素的保护作用机制。
1 材料与方法 1.1 材料 1.1.1 实验动物本实验采用雌性4周龄SPF级BALB/c小鼠48只(合格证:20051128-2),体质量18~22 g,购自上海西普尔-必凯实验动物有限公司。动物饲养及实验方案均严格按照宿州学院动物伦理委员会动物实验规范执行。
1.1.2 药物与试剂老鹳草素(纯度≥98%)购自上海源叶生物科技有限公司;LPS购自上海源叶生物科技有限公司;IL-6、IL-1β、TNF-α酶联免疫吸附测定(ELISA)试剂盒购自中国武汉伊莱瑞特生物科技股份有限公司;苏木精、伊红、瑞氏染色液均购自上海碧云天生物技术有限公司。
1.1.3 仪器瑞士infinite多功能酶标仪;Rotina 380R高速冷冻离心机;CX23型生物显微镜;Milli-Q Synthesis型超纯水系统。
1.2 方法 1.2.1 急性肺损伤模型的复制将48只小鼠随机分为6组(n=8):空白组(control组,给予生理盐水)、LPS组(给予生理盐水)、地塞米松组(Dex组,2 mg/kg)、老鹳草素低剂量组(LD组,10 mg/kg)、老鹳草素中剂量组(MD组,20 mg/kg)、老鹳草素高剂量组(HD组,40 mg/kg)。造模前,空白组和LPS组每日灌胃生理盐水,地塞米松组及老鹳草素各剂量组小鼠按0.1 mL/10 g灌胃相应药物,每日1次,连续3 d。第3天给药1 h后,除control组外,其余各组小鼠均于鼻内单次滴注LPS 4 mg/kg建立急性肺损伤模型[9]。
1.2.2 肺损伤指标检测 1.2.2.1 细胞分类计数造模6 h后用异氟烷(35 mg/kg)麻醉小鼠,从眼眶内眦取血0.5 mL,滴于载玻片上,涂片,用瑞氏染液染色,光学显微镜下计数巨噬细胞、中性粒细胞数量;剩余血液收集于1.5 mL EP管,于4 ℃,4 500 r/min离心15 min,收集上清液,于-80 ℃冰箱保存;血液采集完毕后处死小鼠,分离气管,收集肺泡灌洗液(bronchoalveolar lavage fluid,BALF)[9]。于4 ℃,2 000 r/min离心10 min,收集上清液,离心所得细胞沉淀重悬后取10 μL用于细胞涂片,晾干后用瑞氏染液染色,光学显微镜下计数巨噬细胞、中性粒细胞数量[10]。
1.2.2.2 肺湿干(wet-to-dry,W/D)质量比测定实验结束时,用异氟烷(35 mg/kg)麻醉小鼠,打开小鼠胸腔,取小鼠右肺。分离气管和食管,取右肺叶,立即测量湿质量。随后,将肺在60 ℃下干燥72 h以去除所有水分,然后称量干燥的肺并计算肺的W/D质量比[11]。
1.2.2.3 肺HE染色肺固定于4%福尔马林溶液中,石蜡包埋,切成4 μm切片,置于玻片上进行HE染色[12]。
1.2.2.4 细胞因子测定取血清及BALF上清液,按照ELISA试剂盒说明书,分别加入各个试剂,根据标准曲线与光密度值[D(450)]计算各组上清液中炎症因子IL-6、IL-1β、TNF-α水平。
1.2.2.5 免疫组化检测小鼠肺组织p-NF-κBp65的表达肺组织用4%多聚甲醛固定、石蜡包埋、切片。二甲苯和脱水乙醇中脱蜡,柠檬酸钠缓冲液中微波处理,PBS洗涤。内源性过氧化物酶活性被3%过氧化氢阻断20 min。每个样品用5%山羊血清封闭20 min后,用p-NF-κBp65 (1∶200)抗体处理,置于4 ℃过夜。次日每个样品用PBS洗涤3次,山羊抗兔IgG二抗处理20 min,再用PBS洗涤3次,用3-3'二氨基联苯胺(DAB)染色,然后用苏木精染色。脱水、干燥后用中性胶固定并用显微镜观察。
1.3 统计学处理数据以x±s形式表示。使用SPSS 22.0统计学软件分析。采用Student's t检验对两组之间的差异进行显著性评估,并使用单向方差分析(ANOVA)对两组以上的差异进行评估。P < 0.05为差异具有统计学意义。
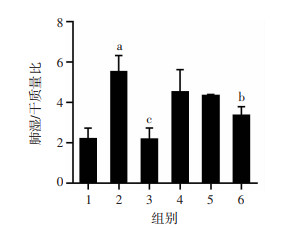
2 结果 2.1 老鹳草素对LPS诱导的急性肺损伤小鼠肺W/D的影响肺水肿程度可通过肺W/D质量比来评估。如图 1所示,LPS组W/D质量比明显高于control组,提示LPS诱导小鼠发生肺水肿(P < 0.01)。与LPS组相比,LD组、MD组的W/D并没有明显降低,但Dex组及HD组的W/D质量比明显降低(P < 0.05)。
|
| 1:空白组;2:LPS组;3:Dex组;4:LD组;5:MD组;6:HD组 a:P < 0.01,与空白组比较;b:P < 0.05,与LPS组比较;c:P < 0.01,与LPS组比较 图 1 老鹳草素对LPS诱导的急性肺损伤小鼠肺组织W/D质量比的影响(n=8,x±s) |
2.2 老鹳草素对LPS诱导的急性肺损伤小鼠肺组织病理学变化的影响
Control组肺泡结构完整,无明显病理改变(图 2);而在LPS组中,炎性细胞浸润严重,肺泡间隔增厚,肺泡腔变小,肺泡水肿、充血;在Dex组和LD、MD、HD组,炎性细胞浸润减轻,不同剂量的老鹳草素减轻了肺损伤,炎性细胞浸润程度降低,这些结果均说明老鹳草素对LPS诱导的肺损伤有保护作用。

|
| 图 2 HE染色观察老鹳草素对LPS诱导的急性肺损伤小鼠肺组织病理形态的影响(×100) |
2.3 老鹳草素对小鼠血液中巨噬细胞和中性粒细胞的数量的影响
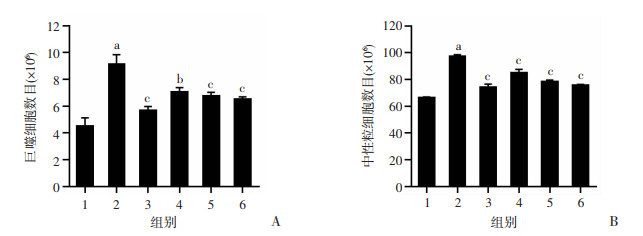
与control组相比,LPS组中的巨噬细胞和中性粒细胞数量明显上升(P < 0.01);与LPS组相比,Dex组和LD、MD、HD组显著降低急性肺损伤小鼠血液中巨噬细胞和中性粒细胞数量(图 3)。
|
| 1:空白组;2:LPS组;3:地塞米松组;4:LD组;5:MD组;6:HD组 a:P < 0.01,与空白组比较;b:P < 0.05,与LPS组比较;c:P < 0.01,与LPS组比较 图 3 老鹳草素对LPS诱导急性损伤小鼠血液中巨噬细胞(A)和中性粒细胞(B)数目的影响(x±s,n=8) |
2.4 老鹳草素对小鼠BALF中巨噬细胞和中性粒细胞的数量的影响
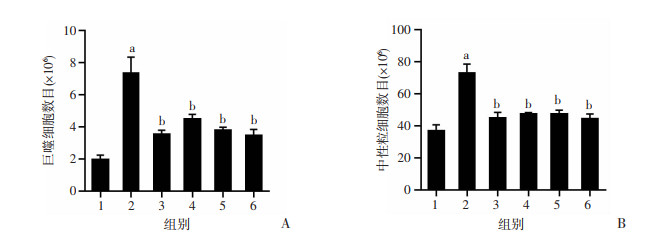
如图 4所示,与control组相比,LPS组中的巨噬细胞和中性粒细胞数量明显上升(P < 0.01);与LPS组相比,Dex组和LD、MD、HD组小鼠BALF中巨噬细胞和中性粒细胞数量均显著降低(P < 0.01)。
|
| 1:空白组;2:LPS组;3:地塞米松组;4:LD组;5:MD组;6:HD组 a:P < 0.01,与空白组比较;b:P < 0.01,与LPS组比较 图 4 老鹳草素对LPS诱导急性损伤小鼠BALF中巨噬细胞(A)和中性粒细胞(B)数目的影响(x±s,n=8) |
2.5 老鹳草素对LPS诱导的急性肺损伤小鼠血清及BALF中TNF-α、IL-1β及IL-6的影响
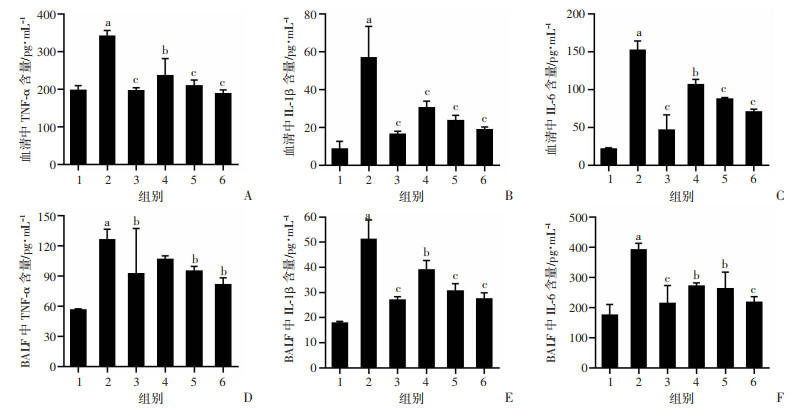
血清及BALF中促炎细胞因子TNF-α、IL-1β及IL-6的水平是反映肺组织炎症程度的重要指标。如图 5所示,与control组相比,LPS组血清及BALF中TNF-α、IL-1β及IL-6的含量显著增加(P < 0.01);给予老鹳草素预处理后,与LPS比较,Dex组和LD、MD、HD组小鼠血清及BALF TNF-α、IL-1β及IL-6的含量明显降低(P < 0.05)。
|
| 1:空白组;2:LPS组;3:地塞米松组;4:LD组;5:MD组;6:HD组 a:P < 0.01,与空白组比较;b:P < 0.05,与LPS组比较;c:P < 0.01,与LPS组比较 图 5 老鹳草素对LPS诱导急性损伤小鼠血清及BALF中TNF-α、IL-1β及IL-6含量的影响(x±s,n=8) |
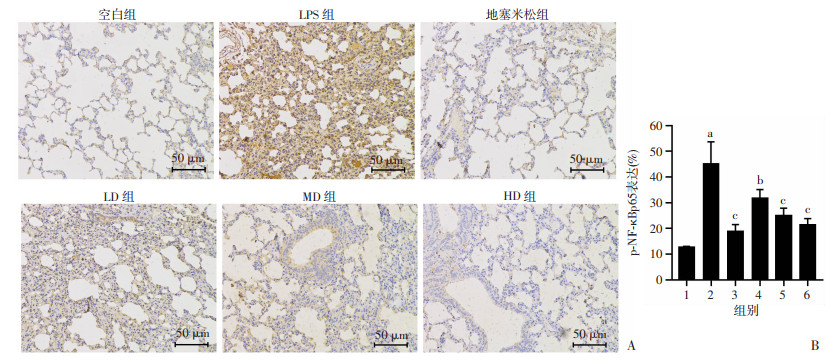
2.6 老鹳草素对LPS诱导急性肺损伤小鼠肺组织p-NF-κBp65的影响
如图 6所示,与control组比较,LPS组中的肺组织p-NF-κBp65表达显著增加(P < 0.01);与LPS组比较,Dex组和LD、MD、HD组小鼠肺组织中p-NF-κBp65表达显著降低(P < 0.05)。
|
| A:HE染色观察各组小鼠肺组织p-NF-κBp65免疫组化染色;B:p-NF-κBp65免疫组化染色定量分析 1:空白组;2:LPS组;3:地塞米松组;4:LD组;5:MD组; 6:HD组 a:P < 0.01,与空白组比较;b:P < 0.05,与LPS组比较;c:P < 0.01,与LPS组比较 图 6 免疫组化法检测老鹳草素对急性肺损伤小鼠肺组织p-NF-κBp65表达的影响(x±s,n=8) |
3 讨论
ALI是一种严重的呼吸疾病,其引起的机体炎症反应会进一步损伤肺组织,其发生率和病死率均较高。而革兰阴性菌感染是ALI最常见的也是最重要的致病因素之一[13-14]。LPS是建立ALI动物模型常用的诱导剂[15-16]。LPS可通过激活中性粒细胞,诱导机体大量炎症因子生成,并释放炎性介质触发炎症反应[17]。目前ALI的临床疗效并不显著,需寻找治疗ALI的有效药物。有研究报道,老鹳草素具有抗炎、抗病毒、抗骨质疏松等作用[6]。但关于老鹳草素对于ALI的作用及作用机制的研究鲜少报道。本文通过鼻滴LPS构建急性肺损伤小鼠模型研究老鹳草素对于ALI作用。
由小鼠肺组织的HE染色结果显示空白组小鼠肺组织结构正常,未见明显的病理改变,但LPS组小鼠肺组织出现了明显的损伤和炎症表现,肺泡腔内有大量炎性细胞浸润,肺泡结构完整性被破坏,部分肺泡出现塌陷,而老鹳草素组和Dex组小鼠上述症状明显减轻。老鹳草素预处理还可抑制LPS导致的小鼠肺湿干质量比的增加,肺湿干质量比客观上反映了毛细管通透性的相对程度,是衡量肺水肿的重要指标[18-19]。由上述结果可知,老鹳草素可通过降低肺微血管通透性,改善肺微循环,减轻LPS导致的肺水肿,而对肺组织起到一定的保护作用。
ALI早期症状主要表现为炎症反应,各种炎症因子促进ALI的发展[20-21]。ALI发生时肺泡巨噬细胞和上皮细胞分泌大量的IL-1β,IL-1β促进中性粒细胞和巨噬细胞向血管及肺泡中募集,进而损伤血管内皮细胞及肺泡上皮细胞,导致肺泡毛细血管通透性增加,致肺水肿,其是炎症诱导的重要中介物[22]。IL-1β通过下游细胞因子TNF-α及IL-6发挥作用;TNF-α是重要的启动因子,引发“瀑布效应”[23];IL-6是一种具有中枢调节作用的多功能生长因子,单核巨噬细胞是其主要来源,能促进炎症发展,影响巨噬细胞的生长和分化,在炎症方面发挥关键作用。血清及BALF中TNF-α、IL-1β及IL-6的含量均能反映炎症的严重程度。本研究结果显示,LPS组血液及BALF中巨噬细胞及中性粒细胞数目明显增加,血清及BALF中炎症因子TNF-α、IL-1β及IL-6的含量明显增加,而Dex及老鹳草素的预处理能显著降低血液及BALF中巨噬细胞及中性粒细胞数目,并能显著降低血清及BALF中炎症因子TNF-α、IL-1β及IL-6的含量,说明老鹳草素有良好的抗炎活性,对LPS诱导的促炎性细胞因子的释放有明显的抑制作用。
NF-κB即核转录因子,也是促进急性肺损伤发展的一个重要的调控蛋白,可参与调节机体的各种反应。NF-κB能通过调控机体分泌多种促炎因子,包括TNF-α、IL-1β、IL-10、IL-6等,来破坏肺泡毛细血管的完整性,诱导大批的单核-巨噬细胞、中性粒细胞聚集于肺部,加重炎症的发生,导致肺水肿。本实验结果显示:与control组比较,LPS组NF-κB表达水平明显升高;与LPS组比较,老鹳草素的预处理能明显降低p-NF-κBp65的表达,这与炎症因子、肺组织的病理学变化结果相一致,说明老鹳草素可通过调控p-NF-κBp65蛋白的表达减少巨噬细胞、中性粒细胞的聚集,减少炎性细胞因子的释放、改善肺组织损伤。
| [1] |
BELLANI G, PHAM T, LAFFEY J, et al. Incidence of acute respiratory distress syndrome: reply[J]. JAMA, 2016, 316(3): 347. |
| [2] |
BAO H, GAO F Y, XIE G G, et al. Angiotensin-converting enzyme 2 inhibits apoptosis of pulmonary endothelial cells during acute lung injury through suppressing miR-4262[J]. Cell Physiol Biochem, 2015, 37(2): 759-767. |
| [3] |
JIA H P, SODHI C P, YAMAGUCHI Y, et al. Pulmonary epithelial TLR4 activation leads to lung injury in neonatal necrotizing enterocolitis[J]. J Immunol, 2016, 197(3): 859-871. |
| [4] |
ROCCO P R M, NIEMAN G F. ARDS: what experimental models have taught us[J]. Intensive Care Med, 2016, 42(5): 806-810. |
| [5] |
JIANG K F, GUO S, YANG C, et al. Barbaloin protects against lipopolysaccharide (LPS)-induced acute lung injury by inhibiting the ROS-mediated PI3K/AKT/NF-κB pathway[J]. Int Immunopharmacol, 2018, 64: 140-150. |
| [6] |
李倩, 买吾拉江·阿不都热衣木, 徐芳, 等. 老鹳草素药理研究进展[J]. 中国中医药信息杂志, 2016, 23(8): 125-128. LI Q, ABUDUREYIMU M W L J, XU F, et al. Research progress in pharmaceutical activities of geraniin[J]. Chin J Inf Tradit Chin Med, 2016, 23(8): 125-128. |
| [7] |
KANG K A, LEE I K, ZHANG R, et al. Radio protective effect of geraniin via the inhibition of apoptosis triggered by γ-radiation-induced oxidative stress[J]. Cell Biol Toxicol, 2011, 27(2): 83-94. |
| [8] |
LI J Y, HUANG H, FENG M Q, et al. In vitro and in vivo anti-hepatitis B virus activities of a plant extract from Geranium carolinianum L[J]. Antiviral Res, 2008, 79(2): 114-120. |
| [9] |
曹志敏, 唐明美, 文强, 等. 内毒素所致急性肺损伤动物模型的研究进展[J]. 实验动物科学, 2017, 34(1): 62-65, 70. CAO Z M, TANG M M, WEN Q, et al. Research progress of endotoxin-induced ALI models[J]. Lab Animal Sci, 2017, 34(1): 62-65, 70. |
| [10] |
刘畅, 程晓丹, 孙家安, 等. 绿原酸通过调控miR-223/NLRP3轴减轻脂多糖诱导的小鼠急性肺损伤的机制[J]. 中南大学学报(医学版), 2022, 47(3): 280-288. LIU C, CHENG X D, SUN J A, et al. Mechanism of chlorogenic acid reducing lipopolysaccharide-induced acute lung injury in mice by regulating miR-223/NLRP3 axis[J]. J Central South Univ Med Sci, 2022, 47(3): 280-288. |
| [11] |
秦臻, 汪勃, 谭赵霞, 等. 姜黄素抑制TLR4/HMGB1通路保护脂多糖诱导急性肺损伤的作用[J]. 中国胸心血管外科临床杂志, 2020, 27(6): 685-688. QIN Z, WANG B, TAN Z X, et al. Curcumin inhibits toll-like receptor 4/high mobility group box 1 pathway to protect lipopolysaccharide-induced acute lung injury[J]. Chin J Clin Thorac Cardiovasc Surg, 2020, 27(6): 685-688. |
| [12] |
邱建磊. 谷氨酰胺对机械通气肺损伤的保护作用及机制[D]. 济南: 山东大学, 2018. QIU J L. Theprotective effect and mechanisms of glutamine on ventilator-induced lung injury[D]. Jinan: Shandong University, 2018. |
| [13] |
BRACKETT D J, LERNER M R, LACQUEMENT M A, et al. A synthetic lipopolysaccharide-binding peptide based on the neutrophil-derived protein CAP37 prevents endotoxin-induced responses in conscious rats[J]. Infect Immun, 1997, 65(7): 2803-2811. |
| [14] |
WANG J, LIU Y T, XIAO L, et al. Anti-inflammatory effects of apigenin in lipopolysaccharide-induced inflammatory in acute lung injury by suppressing COX-2 and NF-kB pathway[J]. Inflammation, 2014, 37(6): 2085-2090. |
| [15] |
BAYSTON K F, COHEN J. Bacterial endotoxin and current concepts in the diagnosis and treatment of endotoxaemia[J]. J Med Microbiol, 1990, 31(2): 73-83. |
| [16] |
MENDEN H, WELAK S, COSSETTE S, et al. Lipopolysaccharide (LPS)-mediated angiopoietin-2-dependent autocrine angiogenesis is regulated by NADPH oxidase 2 (Nox2) in human pulmonary microvascular endothelial cells[J]. J Biol Chem, 2015, 290(9): 5449-5461. |
| [17] |
RAHMAN I, SKWARSKA E, MACNEE W. Attenuation of oxidant/antioxidant imbalance during treatment of exacerbations of chronic obstructive pulmonary disease[J]. Thorax, 1997, 52(6): 565-568. |
| [18] |
DUANY L, LEAROYD J, MELITON A Y, et al. Inhibition of Pyk2 blocks lung inflammation and injury in a mouse model of acute lung injury[J]. Respir Res, 2012, 13(1): 4. |
| [19] |
WANG J, MA C H, WANG S M. Effects of acteoside on lipopolysaccharide-induced inflammation in acute lung injury via regulation of NF-κB pathway in vivo and in vitro[J]. Toxicol Appl Pharmacol, 2015, 285(2): 128-135. |
| [20] |
LI Y C, YEH C H, YANG M L, et al. Luteolin suppresses inflammatory mediator expression by blocking the Akt/NFκB pathway in acute lung injury induced by lipopolysaccharide in mice[J]. Evid Based Complement Alternat Med, 2012, 2012: 383608. |
| [21] |
DOLINAY T, KIM Y S, HOWRYLAK J, et al. Inflammasome-regulated cytokines are critical mediators of acute lung injury[J]. Am J Respir Crit Care Med, 2012, 185(11): 1225-1234. |
| [22] |
YOSHINARI D, TAKEYOSHI I, KOIBUCHI Y, et al. Effects of a dual inhibitor of tumor necrosis factor-alpha and interleukin-1 on lipopolysaccharide-induced lung injury in rats: involvement of the p38 mitogen-activated protein kinase pathway[J]. Crit Care Med, 2001, 29(3): 628-634. |
| [23] |
SUSANTITAPHONG P, PERIANAYAGAM M C, TIGHIOUART H, et al. Tumor necrosis factor alpha promoter polymorphism and severity of acute kidney injury[J]. Nephron Clin Pract, 2013, 123(1/2): 67-73. |




