人类的感官机制一直激发科学家们的好奇心,人有五种感觉:视觉、听觉、嗅觉、味觉、触觉,“眼耳鼻舌身意,色声香味触法”,都是诺奖级的研究。1961年,美籍匈牙利裔科学家Georg von Bekesy发现听觉器官耳蜗及其作用,1967年,美国科学家George Wald通过生物化学手段揭示rhodopsin感光的生化反应,2004年,美国科学家Richard Axe和Linda B. Buck揭示了嗅觉受体分子家族,上述工作分别获得了诺贝尔生理学或医学奖。今年的诺贝尔生理学或医学奖授予温度感知与触觉研究的美国科学家David Julius和Ardem Patapoutian,前者发现了对辣椒素有强烈响应的基因TRPV1,并证实其对温度感知的作用;同时,他与Ardem Patapoutian分别独立发现对寒冷反应的受体TRPM8。此外,2000年,Ardem Patapoutian还发现了应对机械刺激的触觉受体PIEZO。对TRPV1和TRPM8的早期研究主要围绕其对温度和痛觉的感知,但TRPV1和TRPM8在全身组织器官有广泛分布,提示其有许多非神经感知的作用,这也一直吸引着国内外学者进行不断探索。近年的研究显示,TRPV1和TRPM8的功能异常与许多疾病有关,也是许多疾病干预的靶点。我们团队自2004年始从我国流行病学调查结果中发现,心血管和代谢病的罹患率存在很大的地区差异,并与不同区域的饮食习惯关联,尤其是辣膳食中的辣椒素和薄荷叶中的薄荷醇(menthol),均有相应的分子靶标TRPV1和TRPM8。为此,我们对TRPV1和TRPM8在心血管及代谢功能调控中的作用及其与心血管和代谢病的关系开展了系列研究。本文主要聚焦TRPV1和TRPM8对心血管和糖脂代谢的调控,结合本团队工作及相关的研究进展,阐述TRPV1和TRPM8在重大慢病发生中的作用,以期为重大慢病的防治提供新思路。
1 TRPV1和TRPM8的分布及功能TRPV1和TRPM8属瞬时受体电位(transient receptor potential, TRP)通道家族,TRP通道是一类非选择性的阳离子通道,广泛分布于哺乳动物的多种组织和细胞。根据氨基酸序列的不同,TRP通道可分为6个亚家族:TRPC(canonical;TRPC1-7)、TRPV(vanilloid;TRPV1-6)、TRPP(polycystin;TRPP2,TRPP3,TRPP5)、TRPA(ankyrin;TRPA1)、TRPML(mucolipin;TRPML1-3)和TRPM(melastatin;TRPM1-8)[1]。TRP通道由6个跨膜结构域及胞内的N末端和C末端组成,靠近C末端的TM5和TM6形成离子微孔区,是决定其温度、电压和配体敏感性的关键部位。TRP通道是“细胞传感器”,对细胞环境的变化作出反应,包括温度、拉伸/压力、化学物质、氧化/还原、渗透压和pH值[2]。
TRPV1作为TRP通道家族中TRPV(vanilloid)亚家族成员,是一种可通过钠、钙、氢等阳离子的通道,人源TRPV1由838个氨基酸组成,TRPV1通道常以四聚体形式定位于细胞膜上,在线粒体膜上也有TRPV1通道表达。与电压依赖的离子通道不同,TRPV1通道无典型的电压感受器,其主要被热(>42 ℃)、酸性(pH < 6)、辣椒素和其他植物来源的香草素等激活。此外,机体内源性物质如花生四烯酸代谢物、过氧化氢、一氧化氮(NO)等也可激活TRPV1[2]。TRPV1蛋白翻译后的磷酸化对其功能的发挥至关重要。蛋白激酶A(PKA)和钙/钙调蛋白依赖性蛋白激酶Ⅱ(CAMKⅡ)可磷酸化TRPV1使其活性增加,而钙调磷酸酶使TRPV1去磷酸化可导致通道脱敏[3-4]。此外,磷脂酶C水解磷脂酰肌醇-4, 5-二磷酸(PIP2)后也使TRPV1活性增加[5]。在神经系统,TRPV1通过钙信号或胞膜去极化,介导机体对内外源性化学刺激和温度等物理刺激做出反应,引起伤害信号向神经中枢传递并最终产生痛觉。
有研究利用cDNA文库发现一种与TRP家族高度同源的基因并命名为TRP-p8[1],TRP-p8可被冷环境(8~28 ℃)、薄荷醇(menthol)、桉油素和icilin等冷却剂所激活,产生凉爽感觉,将其定为冷和薄荷醇敏感受体(CMR或TRPM8)[6]。人类TRPM8基因定位于2号染色体上(2q37.1),全长约102.12 kb,由24个外显子组成。TRPM8蛋白分布较为广泛,在外周背根神经节(DRG)和中枢三叉神经元均有表达。此外,TRPM8在血管平滑肌、肺、骨骼肌、肠系膜、胃底、肝脏、膀胱、前列腺、乳腺、胸腺和皮肤等组织器官均有分布[7]。TRPM8除在细胞膜表达外,还存在于肌浆网和内质网。我们的研究证实TRPM8在单核细胞及线粒体均有表达,激活TRPM8可促进单核细胞的胞外钙内流。TRPM8对Ca2+具有良好的通透性,通过影响细胞内钙信号调节细胞内钙库释放及钙相关激酶活性等,发挥冷感知等生物学作用。生理情况下,TRPM8作为一种冷刺激感受通道传导无伤害的冷刺激,启动冷感知从而实现体温调节。TRPM8还参与调控疼痛感觉,其介导的冷刺激信号是神经性疼痛的潜在治疗靶点[7]。但TRPV1和TRPM8在心血管、肝脏、脂肪、肾脏、肌肉和胰腺等组织器官也有丰富的表达,提示其除神经感知外,在其他组织器官还有作用。
2 TRPV1和TRPM8对心血管的作用TRPV1是所有TRP通道中研究较多的亚型之一,TRPV1在心脏交感传入纤维、心肌细胞和成纤维细胞均有表达。TRPV1基因敲除小鼠心肌TGF-β、Smad2、VEGF和MMP-2表达升高,增加心脏纤维化,并减少心肌保护蛋白降钙素基因相关肽(CGRP)和P物质的产生[8]。TRPV1基因敲除小鼠冠状动脉结扎后梗死面积和死亡率增加,炎症细胞浸润、毛细血管密度和胶原含量显著升高, 激活TRPV1可抑制心肌缺血损伤后梗死区域的炎症和病理性心脏重塑[9]。TRPV1在主动脉结扎诱导的心肌肥厚小鼠中表达上调,TRPV1基因敲除与心肌肥厚有关[10]。激活TRPV1对心肌缺血再灌注损伤的心功能具有保护作用,其机制与激活蛋白酶激活受体2,通过PKA/PKC,增强CGRP和SP的释放,抑制炎症反应有关[11]。我们的研究表明,长期高盐饮食可导致小鼠心肌肥厚、纤维化及运动耐量下降,予以膳食辣椒素干预可拮抗高盐导致的心肌重塑,改善心功能,其机制与激活心肌TRPV1改变PPAR-δ/UCP2水平及改善小鼠心肌线粒体功能有关[12-13]。激活TRPV1可增加辣椒素敏感的神经释放CGRP和内皮细胞释放NO,降低血压[14]。辣椒素激活血管内皮TRPV1促进Ca2+内流,增加蛋白激酶A和一氧化氮合成酶(eNOS)的磷酸化,从而促进内皮细胞产生NO[15]。遗传性高血压大鼠予以膳食辣椒素干预可激活血管TRPV1,促进内皮细胞NO合成,改善血管舒张功能,降低血压[16]。TRPV1还参与高盐诱导的高血压发生,对新生幼鼠,化学损毁辣椒素敏感神经,可增加成年鼠对盐的敏感性和升高血压。Dahl盐敏感大鼠TRPV1表达和功能缺失可导致高盐负荷后血压升高。长期膳食辣椒素干预激活TRPV1可改善高盐摄入导致的血管内皮功能障碍及夜间血压升高[17]。TRPV1在肾皮质和肾小管均有表达,我们发现激活TRPV1可提高肾小球滤过率,促进肾脏水、钠排泄。TRPV1功能障碍可导致肾脏排泄功能受损。长期高盐摄入可损伤肾皮质集合管TRPV1通道的功能,膳食辣椒素干预可激活TRPV1抑制集合管上皮钠通道的活性,减少尿钠重吸收,促进尿钠排泄,从而改善高盐诱导的血压升高[18]。此外,我们还证实辣椒素可增强中枢对盐摄入的感知,这种作用有助于减少高盐摄入及盐敏感性高血压的发生[19]。
TRPM8在心脏、血管平滑肌和内皮等广泛表达,也存在于质膜、高尔基体和肌浆网[20-21]。激活TRPM8可减少缺血再灌注导致的心脏梗死面积及心肌丙二醛(MDA)、乳酸脱氢酶(LDH),抑制RhoA和ROCK2表达,而TRPM8拮抗剂可抑制这些作用[22]。膳食薄荷醇激活TRPM8可减轻心肌梗死损伤,降低血浆心肌肌钙蛋白Ⅰ水平,减少梗死面积和及胶原沉积,改善心功能[23]。此外,薄荷醇干预可减少心肌梗死小鼠的炎症因子和趋化因子表达,增加CGRP的释放[23]。在肾血管性高血压模型,血管紧张素Ⅱ(Ang Ⅱ)可抑制血管平滑肌TRPM8表达,薄荷醇激活TRPM8可减少Ang Ⅱ诱导的活性氧和H2O2产生,抑制Ang Ⅱ诱导的NADPH氧化酶NOX1和NOX4上调,抑制Ang Ⅱ介导的RhoA相关蛋白激酶,改善血管结构及功能。此外,TRPM8在肺动脉内皮细胞中表达,其功能受损可导致肺动脉血管反应性增强[24],在肺动脉高压大鼠,激活TRPM8可舒张肺动脉[25],TRPM8作用于GTPase Rap1抑制内皮细胞迁移[26]。我们的研究表明,TRPM8在血管平滑肌细胞膜和肌浆网上均有表达,长期膳食薄荷醇可激活野生型小鼠血管TRPM8,抑制肌浆网钙释放和RhoA/ROCK-2激酶活性及pMYPT-1表达,减少血管收缩反应,从而降低血压,但薄荷醇对TRPM8基因敲除小鼠无此作用[27]。Ang Ⅱ和冷应激诱导高血压小鼠的研究显示,长期膳食薄荷醇干预通过激活肌浆网上的TRPM8,抑制血管紧张素Ⅱ介导的ROS过度生成,减少细胞外Ca2+内流,激活RhoA/ROCK-2激酶,拮抗高血压的发生[21]。
3 TRPV1和TRPM8对糖脂代谢的作用我们首次发现TRPV1在小鼠及人白色脂肪组织(white adipose tissue, WAT)中表达,激活脂肪细胞TRPV1增加细胞钙信号,通过抑制PPARγ和脂肪酸合酶减少脂质生成。辣椒素激活脂肪TRPV1增加Cx43介导的脂肪细胞间Ca2+内流,从而促进脂肪分解[28]。TRPV1在棕色脂肪组织(brown adipose tissue, BAT)和脂肪细胞系有表达,激活TRPV1可上调产热基因表达,诱导3T3-L1前脂肪细胞分化为褐色脂肪表型[29]。TRPV1还可通过控制食欲激素水平或调节胃肠道迷走神经传入信号影响食欲。辣椒素在胃肠道中可直接与TRPV1结合产生传入信号,传递至中枢神经系统下丘脑腹内侧核,促进β2-肾上腺素受体的表达和PRDM16蛋白的产生,从而促进米色脂肪细胞的生成,增加全身能量消耗[30]。口服辣椒素可激活胃肠道内感觉神经的TRPV1,增加支配BAT的交感神经活性,诱导BAT介导的核心温度升高,增加全身能量消耗,降低体脂。在敲除TRPV1或偶联蛋白1(uncoupling protein 1,UCP1)基因的小鼠中,辣椒素的产热和减脂作用减弱[31],我们还发现,激活TRPV1增加BAT中Sirt-1表达,促进其乙酰化和PPARγ与PRDM-16的相互作用,增加能量消耗[32]。在血糖调控方面,TRPV1通过在支配胰岛的传入感觉神经中表达来调节胰岛素分泌。辣膳食可增加人血浆胰高血糖素样肽-1(GLP-1)水平,降低血饥饿素水平。TRPV1在大鼠胰岛β细胞和β细胞系中也有表达,辣椒素可剂量依赖性增加胰岛素分泌,TRPV1拮抗剂可阻断这种作用。我们发现TRPV1受体与GLP-1在肠STC-1细胞有共表达,激活TRPV1通过Ca2+依赖机制促进GLP-1释放。予辣椒素可促进小鼠GLP-1分泌,这种作用被TRPV1拮抗剂抑制[33]。此外,抑制GLP-1部分削弱辣椒素引起的胰岛素升高,这表明TRPV1激活通过依赖于GLP-1影响胰岛素分泌和降低血糖。肝脏和肌肉组织在维持机体糖脂代谢稳态中有重要作用,我们发现辣椒素激活肝脏TRPV1可上调UCP2及过氧化物酶体增殖物激活受体δ(PPARδ)表达,增强自噬作用,减少脂肪肝的形成[34-35]。激活肌肉TRPV1增加骨骼肌过氧化物酶体增殖物激活受体γ(PPARγ)共激活因子-1α(PGC-1α)表达,促进脂肪酸氧化和线粒体功能,增强慢肌纤维功能,从而增加运动耐力[36]。有报道辣椒素激活TRPV1还可增加肌细胞钙/钙调蛋白依赖性蛋白激酶激酶2(CAMKK2)和AMP活化蛋白激酶(AMPK)表达,促进肌肉葡萄糖氧化和ATP生成[37]。
我们首先报道棕色脂肪组织有TRPM8表达,薄荷醇或icilin激活TRPM8可诱导棕色脂肪UCP1表达。皮肤薄荷醇处理或冷暴露导致机体核心体温升高,与UCP1表达增加有关,且这种作用是TRPM8依赖的。在冷暴露期间,TRPM8基因敲除小鼠和TRPM8拮抗剂干预的野生型小鼠的核心体温降低。TRPM8基因敲除小鼠还表现出尾部热损失增加,导致体温过低[38]。薄荷醇灌胃可增强小鼠BAT产热和WAT的“棕色化”。TRPM8基因敲除增加小鼠食物摄入,使其体温下降和肥胖。薄荷醇激活TRPM8可诱导脂肪细胞分化和能量消耗增加,并增强线粒体功能的相关基因表达[39]。激活TRPM8可促进棕色脂肪UCP1依赖的产热和胰岛素敏感性,抑制高脂诱导的葡萄糖耐量异常和肥胖。此外,TRPV1激动剂(辣椒素)和TRPM8激动剂(薄荷醇)联合应用可防止高脂诱导的体质量增加,改善糖代谢和脂肪组织增生,促进棕色脂肪产热和糖利用,抑制脂肪和胰岛素抵抗[40]。TRPM8也参与血糖稳态调节,予薄荷醇或icilin激活TRPM8可增加血清胰高血糖素的浓度,而TRPM8阻断剂可消除这种作用。长期服用薄荷醇可防止高脂饲养的动物体质量增加和胰岛素抵抗,以及脂肪组织增生和肝脏甘油三酯的沉积[41]。与野生型小鼠相比较,TRPM8基因敲除小鼠的胰岛素清除率增加。肝脏无TRPM8表达,但肝脏感觉传入神经有TRPM8表达,其介导神经元调控肝脏胰岛素清除发挥作用[42]。小肠TRPM8的表达降低,与肠道菌群组成和多样性改变有关。此外,TRPM8基因在C2C12肌细胞系中有表达,TRPM8激活增加小鼠能量消耗和改善骨骼肌的运动耐力[43]。
4 TRPV1和TRPM8与其他疾病的关系TRPV1和TRPM8的异常表达和/或功能障碍与多种疾病相关,如肾脏病、脑血管病、呼吸疾病(包括慢性咳嗽、哮喘)、病理性疼痛(炎症、内脏、癌症、偏头痛)、间质性膀胱炎、尿失禁、胰腺炎等。
4.1 TRPV1与肾脏病糖尿病肾病与线粒体钙摄入过多导致足细胞蛋白滤过功能受损关系密切,我们发现膳食辣椒素通过激活TRPV1逆转遗传性肥胖糖尿病或链脲佐菌素诱导的糖尿病小鼠的肾脏损害,其机制涉及辣椒素激活TRPV1缓解高血糖诱导的足细胞线粒体功能障碍,减少线粒体相关膜(MAMs)的形成[44]。此外,激活TRPV1对缺血/再灌注急性肾损伤(AKI)有保护作用, 并可改善AKI的预后,其机制可能与支配肾脏的背根神经节的神经元TRPV1通道有关。有报道TRPV1激活对DOCA盐敏感大鼠的慢性肾纤维化有保护作用。目前未见有TRPM8在肾脏分布的报道。
4.2 TRPV1和TRPM8与脑血管病TRPV1在脑基底动脉有表达,我们发现长期膳食辣椒素激活TRPV1能增加脑基底动脉eNOS磷酸化,预防卒中型自发性高血压大鼠脑卒中的发生,并增加生存时间[16]。TRPV1激活在急性脑损伤时保持低体温是一种有效的神经保护策略,辣椒素激活TRPV1通过作用于大脑和/或周围神经元促进体温下降,在脑梗死发生前对神经起保护作用。在新生大鼠中,辣椒素干预对缺氧/缺血引起的脑损伤产生神经保护作用。TRPM8在脑血管和脑实质中均有表达。研究表明,低温期间软脑膜小动脉对内皮依赖性扩张剂缓激肽和谷氨酸的反应降低,而对高碳酸血症和硝普钠(SNP)的反应保持不变,所有血管扩张剂反应在复温后可恢复,提示头部低温没有产生血管内皮损伤。在正常血供时,TRPM8激活可使软脑膜小动脉收缩,这种作用可被TRPM8抑制剂所拮抗。头部低温可通过激活TRPM8介导内皮依赖性脑血管舒张功能,这对脑血管功能有保护作用[45]
4.3 TRPV1和TRPM8与呼吸系统疾病特定刺激可通过激活TRPV1引起支气管收缩, 豚鼠气管加湿热空气过度通气后,引起支气管收缩,肺阻力增加。TRPV1拮抗剂预处理可使增强的肺阻力降低。辣椒素可以部分地减弱气道收缩。哮喘的特征是嗜酸性粒细胞、中性粒细胞、肥大细胞和CD4+T淋巴细胞增多。TRPV1与哮喘密切相关。在哮喘患者的上皮细胞中TRPV1上调,一项儿童哮喘研究报告TRPV1-1585V基因变异与较低的喘息、咳嗽和活动性哮喘风险相关[46]。慢性阻塞性肺病(COPD)最显著的特征之一是炎症细胞浸润,尤其是IL-8和中性粒细胞, 激活TRPV1可能减轻COPD患者的肺部炎症。TRPM8表达于支配肺支气管系统的自主神经中,TRPM8激活可能会增加气道阻力,与寒冷诱导的哮喘和其他肺部疾病的加重有关[47],TRPM8基因多态性与寒冷诱导的哮喘相关[48]。此外,TRPM8还参与寒冷诱导的呼吸道黏液高分泌[49]。
4.4 TRPV1和TRPM8与膀胱功能TRPV1在非传入神经纤维如膀胱组织,特别是尿路上皮细胞、平滑肌细胞(SMC)和间质细胞有表达和功能。TRPV1的表达呈年龄和性别依赖性,且女性的表达量高于男性[50]。野生型小鼠膀胱分离的小动脉中,辣椒素能够诱导细胞钙升高,并伴有收缩,但这种效应在TRPV1基因敲除小鼠中消失[50],TRPV1对膀胱微循环有调节作用,尤其对女性膀胱微循环有重要影响。在泌尿生殖系统中,TRPM8参与膀胱收缩和膀胱机械压力感知。TRPM8激动剂薄荷醇和icilin予膀胱内而非血管内给药,可增强卡巴酚诱导的离体膀胱收缩,与尿路上皮TRPM8受体激活介导ATP释放有关[51]。膀胱充盈中传入通路的激活也与TRPM8有关[52]。TRPM8还可调节前列腺上皮细胞的增殖、凋亡、离子和蛋白质分泌等[53]。
4.5 TRPV1和TRPM8与皮肤功能皮肤是人体最大的器官,TRPV1在多种皮肤细胞中表达,包括表皮角质形成细胞、肥大细胞、朗格汉斯细胞和皮脂细胞。TRPV1参与调节角质形成细胞的生长和分化[54]。在培养的角质形成细胞中,TRPV1介导的细胞钙可抑制细胞增殖,增加凋亡[55]。此外,热和紫外线能够促进人皮肤中TRPV1的表达。TRPV1介导热和紫外线诱导的人表皮角质形成细胞胶原降解及MMP-1的表达,参与热和紫外线相关的皮肤老化及损伤[56]。
4.6 TRPV1和TRPM8与癌症近年来,研究发现TRPV1在多种类型癌细胞中表达水平发生改变,TRPV1在肿瘤发生、发展中发挥着重要作用。有报道TRPV1与癌细胞增殖、细胞死亡和转移之间有关联。目前约有24种TRPV1激动剂/拮抗剂正在进行临床抗肿瘤试验[57]。最近研究表明, TRPM8也与肿瘤的发生和发展有关, TRPM8在下述几种肿瘤中表达异常, 如前列腺肿瘤、黑色素瘤、乳腺腺癌、膀胱癌和结肠直肠癌, 使其成为潜在的诊断标志物及癌症治疗靶标[58]。
5 干预TRPV1和TRPM8预防心血管代谢病的人群研究我国流行病学调查表明,西南等食辣地区的高血压、肥胖、急性心肌梗死的患病率,以及急性冠脉综合征的年介入治疗量明显低于吃辣少的北方地区[59],辣膳食对心血管与代谢病有何作用并不清楚。目前国际上心血管病防治指南推荐的膳食疗法主要有美国的终止高血压膳食方案(Dietary Approaches to Stop Hypertension,DASH)[60],该方案强调摄入足够的蔬菜、水果、低脂(或脱脂)奶,以维持足够的钾、镁、钙等离子的摄取,并尽量减少饮食中饱和脂肪酸。DASH饮食的作用机制可能与其富含钾、镁、钙、纤维素及抗氧化物质改善心血管与代谢有关。欧洲推出了地中海膳食方案,强调多吃蔬菜、水果、鱼、海鲜、豆类、坚果类食物,其次才是谷类,尤其提倡用橄榄油,饮适量的红酒等,该膳食方案的益处可能与大量食用橄榄油可降低体内的胆固醇水平,降低血压和血糖水平有关[61]。此外,红酒含有较强的抗氧化物质类黄酮,对心血管也有保护作用。
辣膳食的主要活性成份辣椒素可激活TRPV1,有关荟萃分析显示食辣可增加能量消耗,减少能量摄入,增加脂肪氧化,从而减轻体质量和体脂。摄入辣椒素类似物也可通过激活人棕色脂肪组织增加能量消耗,减轻体质量[62]。此外,近50万的人群研究显示,我国成年人群的肥胖存在地区差异并与辛辣食物摄入有关,食辣地区的肥胖风险减少[63]。对全国31个省市自治区2亿多人次外卖点餐的大数据分析显示,喜辛辣食物与发生糖尿病的风险及空腹和餐后血糖水平呈负相关[64]。China Kadoorie Biobank (CKB) 对近50万人的调查也显示辣食摄入的频度与糖尿病死亡风险呈负相关[65],膳食辣椒与胰岛素抵抗有关,食辣可降低血胰岛素水平[66]。国内外人群调查结果均显示,增加食辣程度与频度可减少高血压风险,并与血压水平呈负相关。辣膳食还可改善非酒精性脂肪肝和血脂水平[67-68]。CKB研究还证实,吃辣的频率越高与缺血性心脏病和脑血管病的致死风险显著负相关[65]。2020年美国心脏年会报道由美国、意大利、伊朗及中国逾57万的人群研究结果显示,常吃辣者心血管死亡风险减少26%, 癌症死亡风险减少23%, 全因死亡风险减少25%[69]。
有关TRPM8和薄荷醇的人群研究证据相对较少。我们发现与安慰剂组相比较,慢性薄荷醇胶囊治疗可降低高血压前期个体的收缩压和舒张压,改善其血管舒张功能[27]。有报道TRPM8基因单核苷酸多态性与俄罗斯人群的血总胆固醇、低密度脂蛋白胆固醇和高密度脂蛋白胆固醇水平有关,也与甘油三酯含量、体质量指数和腰臀比相关[70]。另外,已有较多研究证实降低环境温度能增加棕色脂肪的产热功能,有助于减轻体质量。今后如能在前瞻性临床试验中充分证实干预TRPV1和TRPM8具有改善心血管及代谢紊乱的益处,则将提供一种人群层面低成本、易推广的防治心血管代谢病的新措施。
6 展望TRPV1和TRPM8的发现,不仅揭示了机体感知温度的机制,也丰富了人们对温度敏感的TRPV1和TRPM8在非神经系统中作用的认识,及其与心血管和代谢病等重大慢病的内在关联。我们与国内外学者将温度敏感TRP通道引入了心血管和代谢领域的探索,为心血管代谢病的防治提供了新的思路(图 1)。除在心血管代谢领域外,温度敏感TRP通道在癌症、泌尿、呼吸及消化系统中的作用也引起关注,这些与神经和温度感知关系并不密切的作用,提示了许多新的问题,值得进一步深入研究。另外,近年的一些临床试验结果显示,针对温度敏感TRP通道开发的一些激动剂和抑制剂多用于止痛或抗炎,但其引起的一些副作用如改变体温及增加某些肿瘤的风险也不容忽视[71]。从人群层面和临床实践考量,推荐辣味膳食是一个副作用少、易操作和广覆盖的慢病防治措施。目前辣膳食已写入2020年《中国健康方式预防心血管代谢疾病指南》予以推荐[72],这也是中国率先在国际上提出了这类膳食治疗方案。但需注意的是,有相当部分人群不喜辛辣食物,或不能耐受这类食物引起的不适。最近有报道一些无辛辣味的内酯类化合物与辣椒素有相似的化学结构和生物学效应[73],值得进一步探索。总之,今年的诺奖工作将进一步促进人们对TRPV1和TRPM8作用的关注,也将推动其他多个研究领域的发展。
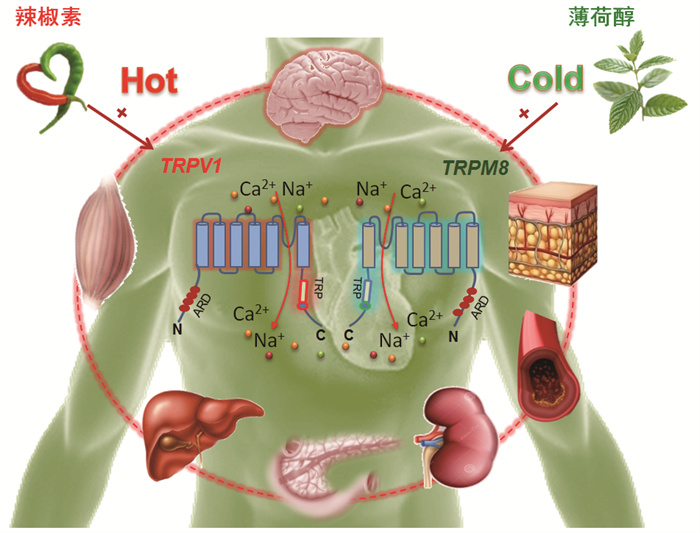
|
| 图 1 TRPV1、TRPM8与心血管和代谢调控 |
| [1] |
TOMINAGA M, CATERINA M J, MALMBERG A B, et al. The cloned capsaicin receptor integrates multiple pain-producing stimuli[J]. Neuron, 1998, 21(3): 531-543. |
| [2] |
SHIMIZU S, TAKAHASHI N, MORI Y. TRPs as chemosensors (ROS, RNS, RCS, gasotransmitters)[J]. Handb Exp Pharmacol, 2014, 223: 767-794. |
| [3] |
BHAVE G, ZHU W, WANG H, et al. cAMP-dependent protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation[J]. Neuron, 2002, 35(4): 721-731. |
| [4] |
NOVAKOVA-TOUSOVA K, VYKLICKY L, SUSANKOVA K, et al. Functional changes in the vanilloid receptor subtype 1 channel during and after acute desensitization[J]. Neuroscience, 2007, 149(1): 144-154. |
| [5] |
PRESCOTT E D, JULIUS D. A modular PIP2 binding site as a determinant of capsaicin receptor sensitivity[J]. Science, 2003, 300(5623): 1284-1288. |
| [6] |
GONZÁLEZ-MUÑIZ R, BONACHE M A, MARTÍN-ESCURA C, et al. Recent progress in TRPM8 modulation: an update[J]. Int J Mol Sci, 2019, 20(11): 2618. |
| [7] |
KANEKO Y, SZALLASI A. Transient receptor potential (TRP) channels: a clinical perspective[J]. Br J Pharmacol, 2014, 171(10): 2474-2507. |
| [8] |
DU Q, LIAO Q, CHEN C, et al. The role of transient receptor potential vanilloid 1 in common diseases of the digestive tract and the cardiovascular and respiratory system[J]. Front Physiol, 2019, 10: 1064. |
| [9] |
HUANG W, RUBINSTEIN J, PRIETO A R, et al. Transient receptor potential vanilloid gene deletion exacerbates inflammation and atypical cardiac remodeling after myocardial infarction[J]. Hypertension, 2009, 53(2): 243-250. |
| [10] |
ZHONG B H, RUBINSTEIN J, MA S T, et al. Genetic ablation of TRPV1 exacerbates pressure overload-induced cardiac hypertrophy[J]. Biomed Pharmacother, 2018, 99: 261-270. |
| [11] |
ZHONG B H, WANG D H. Protease-activated receptor 2-mediated protection of myocardial ischemia-reperfusion injury: role of transient receptor potential vanilloid receptors[J]. Am J Physiol Regul Integr Comp Physiol, 2009, 297(6): R1681-R1690. |
| [12] |
GAO F, LIANG Y, WANG X, et al. TRPV1 activation attenuates high-salt diet-induced cardiac hypertrophy and fibrosis through PPAR-δ upregulation[J]. PPAR Res, 2014, 2014: 491963. |
| [13] |
LANG H, LI Q, YU H, et al. Activation of TRPV1 attenuates high salt-induced cardiac hypertrophy through improvement of mitochondrial function[J]. Br J Pharmacol, 2015, 172(23): 5548-5558. |
| [14] |
RANDHAWA P K, JAGGI A S. TRPV1 channels in cardiovascular system: a double edged sword?[J]. Int J Cardiol, 2017, 228: 103-113. |
| [15] |
YANG D, LUO Z, MA S, et al. Activation of TRPV1 by dietary capsaicin improves endothelium-dependent vasorelaxation and prevents hypertension[J]. Cell Metab, 2010, 12(2): 130-141. |
| [16] |
XU X, WANG P, ZHAO Z, et al. Activation of transient receptor potential vanilloid 1 by dietary capsaicin delays the onset of stroke in stroke-prone spontaneously hypertensive rats[J]. Stroke, 2011, 42(11): 3245-3251. |
| [17] |
HAO X, CHEN J, LUO Z, et al. TRPV1 activation prevents high-salt diet-induced nocturnal hypertension in mice[J]. Pflugers Arch, 2011, 461(3): 345-353. |
| [18] |
LI L, WANG F, WEI X, et al. Transient receptor potential vanilloid 1 activation by dietary capsaicin promotes urinary sodium excretion by inhibiting epithelial sodium channel α subunit-mediated sodium reabsorption[J]. Hypertension, 2014, 64(2): 397-404. |
| [19] |
LI Q, CUI Y, JIN R, et al. Enjoyment of spicy flavor enhances central salty-taste perception and reduces salt intake and blood pressure[J]. Hypertension, 2017, 70(6): 1291-1299. |
| [20] |
MAHIEU F, OWSIANIK G, VERBERT L, et al. TRPM8-independent menthol-induced Ca2+release from endoplasmic reticulum and Golgi[J]. J Biol Chem, 2007, 282(5): 3325-3336. |
| [21] |
XIONG S Q, WANG B, LIN S Y, et al. Activation of transient receptor potential melastatin subtype 8 attenuates cold-induced hypertension through ameliorating vascular mitochondrial dysfunction[J]. J Am Heart Assoc, 2017, 6(8). |
| [22] |
CHENG Q Y, YANG M C, WU J, et al. Reduced cardiac ischemia/reperfusion injury by hypothermic reperfusion via activation of transient receptor potential M8 channel[J]. Life Sci, 2019, 232: 116658. |
| [23] |
WANG Q, YANG Y, CHEN K, et al. Dietary menthol attenuates inflammation and cardiac remodeling after myocardial infarction via the transient receptor potential melastatin 8[J]. Am J Hypertens, 2020, 33(3): 223-233. |
| [24] |
LIU X R, LIU Q, CHEN G Y, et al. Down-regulation of TRPM8 in pulmonary arteries of pulmonary hypertensive rats[J]. Cell Physiol Biochem, 2013, 31(6): 892-904. |
| [25] |
HUANG F, NI M, ZHANG J M, et al. TRPM8 downregulation by angiotensin Ⅱ in vascular smooth muscle cells is involved in hypertension[J]. Mol Med Rep, 2017, 15(4): 1900-1908. |
| [26] |
GENOVA T, GROLEZ G P, CAMILLO C, et al. TRPM8 inhibits endothelial cell migration via a non-channel function by trapping the small GTPase Rap1[J]. J Cell Biol, 2017, 216(7): 2107-2130. |
| [27] |
SUN J, YANG T, WANG P, et al. Activation of cold-sensing transient receptor potential melastatin subtype 8 antagonizes vasoconstriction and hypertension through attenuating RhoA/Rho kinase pathway[J]. Hypertension, 2014, 63(6): 1354-1363. |
| [28] |
CHEN J, LI L, LI Y, et al. Activation of TRPV1 channel by dietary capsaicin improves visceral fat remodeling through connexin43-mediated Ca2+ influx[J]. Cardiovasc Diabetol, 2015, 14: 22. |
| [29] |
BABOOTA R K, SINGH D P, SARMA S M, et al. Capsaicin induces "brite" phenotype in differentiating 3T3-L1 preadipocytes[J]. PLoS One, 2014, 9(7): e103093. |
| [30] |
SAITO M, MATSUSHITA M, YONESHIRO T, et al. Brown adipose tissue, diet-induced thermogenesis, and thermogenic food ingredients: from mice to men[J]. Front Endocrinol, 2020, 11: 222. |
| [31] |
BASKARAN P, KRISHNAN V, REN J, et al. Capsaicin induces browning of white adipose tissue and counters obesity by activating TRPV1 channel-dependent mechanisms[J]. Br J Pharmacol, 2016, 173(15): 2369-2389. |
| [32] |
BASKARAN P, KRISHNAN V, FETTEL K, et al. TRPV1 activation counters diet-induced obesity through sirtuin-1 activation and PRDM-16 deacetylation in brown adipose tissue[J]. Int J Obes (Lond), 2017, 41(5): 739-749. |
| [33] |
WANG P, YAN Z, ZHONG J, et al. Transient receptor potential vanilloid 1 activation enhances gut glucagon-like peptide-1 secretion and improves glucose homeostasis[J]. Diabetes, 2012, 61(8): 2155-2165. |
| [34] |
LI L, CHEN J, NI Y, et al. TRPV1 activation prevents nonalcoholic fatty liver through UCP2 upregulation in mice[J]. Pflugers Arch, 2012, 463(5): 727-732. |
| [35] |
LI Q, LI L, WANG F, et al. Dietary capsaicin prevents nonalcoholic fatty liver disease through transient receptor potential vanilloid 1-mediated peroxisome proliferator-activated receptor δ activation[J]. Pflugers Arch, 2013, 465(9): 1303-1316. |
| [36] |
LUO Z D, MA L Q, ZHAO Z G, et al. TRPV1 activation improves exercise endurance and energy metabolism through PGC-1α upregulation in mice[J]. Cell Res, 2012, 22(3): 551-564. |
| [37] |
VAHIDI FERDOWSI P, AHUJA K D K, BECKETT J M, et al. TRPV1 activation by capsaicin mediates glucose oxidation and ATP production independent of insulin signalling in mouse skeletal muscle cells[J]. Cells, 2021, 10(6): 1560. |
| [38] |
REIMÁNDEZ A, FERNÁNDEZ-PEÑA C, GARCÍA G, et al. Deletion of the cold thermoreceptor TRPM8 increases heat loss and food intake leading to reduced body temperature and obesity in mice[J]. J Neurosci, 2018, 38(15): 3643-3656. |
| [39] |
KHARE P, CHAUHAN A, KUMAR V, et al. Bioavailable menthol (transient receptor potential melastatin-8 agonist) induces energy expending phenotype in differentiating adipocytes[J]. Cells, 2019, 8(5): E383. |
| [40] |
TABUR S, OZTUZCU S, DUZEN I V, et al. Role of the transient receptor potential (TRP) channel gene expressions and TRP melastatin (TRPM) channel gene polymorphisms in obesity-related metabolic syndrome[J]. Eur Rev Med Pharmacol Sci, 2015, 19(8): 1388-1397. |
| [41] |
KHARE P, MANGAL P, BABOOTA R K, et al. Involvement of glucagon in preventive effect of menthol against high fat diet induced obesity in mice[J]. Front Pharmacol, 2018, 9: 1244. |
| [42] |
MCCOY D D, ZHOU L G, NGUYEN A K, et al. Enhanced insulin clearance in mice lacking TRPM8 channels[J]. Am J Physiol Endocrinol Metab, 2013, 305(1): E78-E88. |
| [43] |
LI C, LI J, XIONG X J, et al. TRPM8 activation improves energy expenditure in skeletal muscle and exercise endurance in mice[J]. Gene, 2018, 641: 111-116. |
| [44] |
WHITING S, DERBYSHIRE E J, TIWARI B. Could capsaicinoids help to support weight management? A systematic review and meta-analysis of energy intake data[J]. Appetite, 2014, 73: 183-188. |
| [45] |
FEDINEC A L, LIU J, ZHANG R, et al. The cold receptor TRPM8 activation leads to attenuation of endothelium-dependent cerebral vascular functions during head cooling[J]. J Cereb Blood Flow Metab, 2021, 271678X211018035. |
| [46] |
CHEN C L, LI H, XING X H, et al. Effect of TRPV1 gene mutation on bronchial asthma in children before and after treatment[J]. Allergy Asthma Proc, 2015, 36(2): e29-e36. |
| [47] |
XING H, LING J X, CHEN M, et al. TRPM8 mechanism of autonomic nerve response to cold in respiratory airway[J]. Mol Pain, 2008, 4: 22. |
| [48] |
NAUMOV D E, PERELMAN J M, KOLOSOV V P, et al. Transient receptor potential melastatin 8 gene polymorphism is associated with cold-induced airway hyperresponsiveness in bronchial asthma[J]. Respirology, 2015, 20(8): 1192-1197. |
| [49] |
LI M C, LI Q, YANG G, et al. Cold temperature induces mucin hypersecretion from normal human bronchial epithelial cells in vitro through a transient receptor potential melastatin 8 (TRPM8)-mediated mechanism[J]. J Allergy Clin Immunol, 2011, 128(3): 626-634. |
| [50] |
PHAN T X, TON H T, CHEN Y, et al. Sex-dependent expression of TRPV1 in bladder arterioles[J]. Am J Physiol Renal Physiol, 2016, 311(5): F1063-F1073. |
| [51] |
VAHABI B, PARSONS B A, DORAN O, et al. TRPM8 agonists modulate contraction of the pig urinary bladder[J]. Can J Physiol Pharmacol, 2013, 91(7): 503-509. |
| [52] |
ITO H, AIZAWA N, SUGIYAMA R, et al. Functional role of the transient receptor potential melastatin 8 (TRPM8) ion channel in the urinary bladder assessed by conscious cystometry and ex vivo measurements of single-unit mechanosensitive bladder afferent activities in the rat[J]. BJU Int, 2016, 117(3): 484-494. |
| [53] |
ZHANG L, BARRITT G J. Evidence that TRPM8 is an androgen-dependent Ca2+ channel required for the survival of prostate cancer cells[J]. Cancer Res, 2004, 64(22): 8365-8373. |
| [54] |
HO J C, LEE C H. TRP channels in skin: from physiological implications to clinical significances[J]. Biophysics (Nagoya-Shi), 2015, 11: 17-24. |
| [55] |
TÓTH B I, DOBROSI N, DAJNOKI A, et al. Endocannabinoids modulate human epidermal keratinocyte proliferation and survival via the sequential engagement of cannabinoid receptor-1 and transient receptor potential vanilloid-1[J]. J Invest Dermatol, 2011, 131(5): 1095-1104. |
| [56] |
LEE Y M, KIM Y K, KIM K H, et al. A novel role for the TRPV1 channel in UV-induced matrix metalloproteinase (MMP)-1 expression in HaCaT cells[J]. J Cell Physiol, 2009, 219(3): 766-775. |
| [57] |
LI L, CHEN C, CHIANG C, et al. The impact of TRPV1 on cancer pathogenesis and therapy: a systematic review[J]. Int J Biol Sci, 2021, 17(8): 2034-2049. |
| [58] |
LIU Z, WU H, WEI Z, et al. TRPM8: a potential target for cancer treatment[J]. J Cancer Res Clin Oncol, 2016, 142(9): 1871-1881. |
| [59] |
LI X, WU C, LU J, et al. Cardiovascular risk factors in China: a nationwide population-based cohort study[J]. Lancet Public Health, 2020, 5(12): e672-e681. |
| [60] |
ECKEL R H, JAKICIC J M, ARD J D, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines[J]. Circulation, 2014, 129: S76-S99. |
| [61] |
VITALE M, MASULLI M, CALABRESE I, et al. Impact of a Mediterranean dietary pattern and its components on cardiovascular risk factors, glucose control, and body weight in people with type 2 diabetes: a real-life study[J]. Nutrients, 2018, 10(8): 1067. |
| [62] |
YONESHIRO T, AITA S, KAWAI Y, et al. Nonpungent capsaicin analogs (capsinoids) increase energy expenditure through the activation of brown adipose tissue in humans[J]. Am J Clin Nutr, 2012, 95(4): 845-850. |
| [63] |
SUN D, LV J, CHEN W, et al. Spicy food consumption is associated with adiposity measures among half a million Chinese people: the China Kadoorie Biobank study[J]. BMC Public Health, 2014, 14: 1293. |
| [64] |
ZHAO Z, LI M, LI C, et al. Dietary preferences and diabetic risk in China: a large-scale nationwide Internet data-based study[J]. J Diabetes, 2020, 12(4): 270-278. |
| [65] |
LV J, QI L, YU C Q, et al. Consumption of spicy foods and total and cause specific mortality: population based cohort study[J]. BMJ, 2015, h3942. |
| [66] |
LI J, WANG R, XIAO C. Association between chilli food habits with iron status and insulin resistance in a Chinese population[J]. J Med Food, 2014, 17(4): 472-478. |
| [67] |
YUAN L J, QIN Y, WANG L, et al. Capsaicin-containing chili improved postprandial hyperglycemia, hyperinsulinemia, and fasting lipid disorders in women with gestational diabetes mellitus and lowered the incidence of large-for-gestational-age newborns[J]. Clin Nutr, 2016, 35(2): 388-393. |
| [68] |
SINGH S P, SINGH A, MISRA D, et al. Risk factors associated with non-alcoholic fatty liver disease in indians: a case-control study[J]. J Clin Exp Hepatol, 2015, 5(4): 295-302. |
| [69] |
YAMANI N, MUSHEER A, GOSAIN P, et al. Meta-analysis evaluating the impact of chili-pepper intake on all-cause and cardiovascular mortality: a systematic review[J]. Ann Med Surg (Lond), 2021, 70: 102774. |
| [70] |
POTAPOVA T A, BABENKO V N, KOBZEV V F, et al. Associations of cold receptor TRPM8 gene single nucleotide polymorphism with blood lipids and anthropometric parameters in Russian population[J]. Bull Exp Biol Med, 2014, 157(6): 757-761. |
| [71] |
GARAMI A, SHIMANSKY Y P, RUMBUS Z, et al. Hyperthermia induced by transient receptor potential vanilloid-1 (TRPV1) antagonists in human clinical trials: Insights from mathematical modeling and meta-analysis[J]. Pharmacol Ther, 2020, 208: 107474. |
| [72] |
中华预防医学会, 中华预防医学会心脏病预防与控制专业委员会, 中华医学会糖尿病学分会, 等. 中国健康生活方式预防心血管代谢疾病指南[J]. 中国循环杂志, 2020, 35(3): 209-230. Chinese Preventive Medicine Association, Branch of Heart Disease of Prevention and Control, Diabetes Branch of Chinese Medical Association, et al. Chinese guideline on healthy lifestyle to prevent cardiometabolic diseases[J]. Chin Circ J, 2020, 35(3): 209-230. |
| [73] |
TOBITA N, MAKINO M, FUJITA R, et al. Sweet scent lactones activate hot capsaicin receptor, TRPV1[J]. Biochem Biophys Res Commun, 2021, 534: 547-552. |




