帕金森病(Parkinson’s disease,PD)是全球常见的年龄相关性神经退行性疾病。目前,临床主要通过药物治疗包括抗胆碱能药物、多巴胺替代治疗、多巴胺受体激动剂等来缓解PD患者运动症状,但并不能阻止疾病进展[1]。微小RNA(miRNA)是重要的生物调节分子,其可结合靶mRNA抑制蛋白表达参与调控细胞过程,miRNA的失调可能导致神经退行性疾病、癌症等多种疾病的发生和发展[2-3]。研究报道PD动物模型、细胞模型以及人脑中miR-103a-3p表达明显升高,抑制其表达可改善体内外线粒体吞噬发挥神经保护功能,调节miR-103a-3p水平可能是一种潜在PD治疗策略[4]。含有Src同源结构域2的衔接蛋白3(SHC adaptor protein 3, SHC3)属于Shc家族适配器蛋白,SHC3基因编码p52Shc3和p64Shc3两个亚型。SHC3与细胞存活和分化有关,其下调可导致缺氧信号、细胞凋亡和炎症反应异常改变[5]。SHC3沉默可加重PD大鼠黑质多巴胺神经元氧化应激损伤,导致运动发育迟缓[6]。靶基因预测显示miR-103a-3p与SHC3-3’-非翻译区(UTR)存在结合位点,但miR-103a-3p靶向SHC参与PD进展未见报道。鸢尾苷元是鸢尾科植物射干的重要成分,研究报道鸢尾苷元通过抑制氧化损伤对急性心肌梗死小鼠心肌有明显的保护作用[7]。鸢尾苷元还可抑制促炎症因子产生,降低肝组织损伤和细胞凋亡,对小鼠爆发性肝衰竭具有治疗作用[8]。1-甲基-4-苯基吡啶离子(MPP+)具有神经毒性,其诱导PC12细胞是最常见的PD细胞模型[9]。前期预实验显示鸢尾苷元处理显著降低PD模型细胞中miR-103a-3p表达水平,提高SHC3表达水平。因此,本研究旨在探讨鸢尾苷元对PD模型细胞损伤的保护作用,并以miR-103a-3p/SHC3为切入点分析其可能机制。
1 材料与方法 1.1 材料PC12细胞购自中国科学院上海细胞库;1-甲基-4-苯基吡啶离子(MPP+)购自美国Sigma公司;miR-103a-3p抑制物(Inhibitor)及其阴性对照(anti-miR-NC)、miR-103a-3p模拟物(mimic)、荧光素酶报告基因重组载体购自上海生工生物公司;鸢尾苷元(XY-BZP-2116,纯度99%)购自上海烜雅生物公司;膜联蛋白V-异硫氰酸荧光素(Annexin V-FITC)凋亡检测试剂盒、乳酸脱氢酶(lactate dehydrogenase, LDH)检测试剂盒、超氧化物歧化酶(superoxide dismutase, SOD)活性检测试剂盒、丙二醛(malondialdehyde, MDA)含量检测试剂盒购自北京索莱宝生物公司;Taqman miRNA逆转录试剂盒、TaqMan miRNA定量试剂盒购自美国ABI公司;SHC3兔多抗体(ab233477)、羊抗兔IgG二抗(ab205718)、甘油醛-3-磷酸脱氢酶(GAPDH)兔多抗(ab245355)购自上海艾博抗贸易公司。
1.2 方法 1.2.1 细胞培养、PD模型构建PC12在添加10%胎牛血清、1%青霉素-链霉素混合物的DMEM高糖培养基置于37 ℃、含5% CO2、95%空气的饱和湿度培养箱中培养。细胞80%融合时进行传代,每3 d传代培养1次。将对数期PC12细胞接种96孔板,每板1×104个细胞,用Lipofectamine 2000将anti-miR-NC、miR-103a-3p Inhibitor、miR-103a-3p mimic分别转染50%融合的PC12细胞。参照齐献忠等[9]实验方法用250 mmol/L的MPP+处理PC12细胞24 h建立PD细胞模型,记为PD组。
1.2.2 CCK-8法检测细胞活力将未转染细胞、转染anti-miR-NC细胞、转染miR-103a-3p Inhibitor细胞、转染miR-103a-3p mimic细胞均以5×103个/孔接种96孔板,置于培养基常规孵育24 h。未转染的细胞分为对照组、PD组、PD+鸢尾苷元50 μmol/L组、PD+鸢尾苷元100 μmol/L组、PD+鸢尾苷元200 μmol/L组。转染细胞根据转染序列不同分为PD+anti-miR-NC组、PD+miR-103a-3p Inhibitor组、PD+鸢尾苷元+miR-103a-3p mimic组。取出96孔板,对照组、PD组、PD+anti-miR-NC组、PD+miR-103a-3p Inhibitor组更换为无血清培养,鸢尾苷元处理组分别加入上述浓度的含药培养基预处理1 h。预处理1 h后,除对照组外,其与各组分别加入250 mmol/L的MPP+作用24 h。按照CCK-8试剂盒步骤检测细胞活力,酶标仪检测450 nm处光密度值[D(450)]。
1.2.3 流式细胞术检测凋亡率磷酸盐缓冲液洗涤各处理组细胞2次,将细胞重悬在1×结合缓冲液中,调整细胞密度为2×105个/mL。将500 μL细胞悬液分别加入流式管,依次加入5 μL的Annexin V-FITC和5 μL的PI,室温避光染色。流式细胞仪分析各组细胞凋亡情况。
1.2.4 试剂盒检测细胞内SOD活性、MDA水平以及LDH释放量收集各组细胞至离心管,3 000 r/min离心10 min后分别收集细胞沉淀和上清液。按照LDH检测试剂盒分析细胞培养液中LDH活性以表示各组PC12细胞LDH释放量。同时向5×106个细胞中加入1 mL提取液,超声破碎后,8 000 r/min离心10 min,收集上清置于冰上。按照SOD活性检测试剂盒、MDA含量检测试剂盒步骤分析各组PC12细胞内SOD活性、MDA水平。
1.2.5 RT-qPCR检测miR-103a-3p表达TRIzol试剂提取各组PC12细胞总RNA,并测量RNA浓度和纯度。用TaqMan miRNA反转录试剂盒合成miRNA的cDNA,用TaqMan miRNA定量试剂盒进行RT-qPCR。2-ΔΔCt法分析miR-103a-3p表达水平。miR-103a-3p(76 bp)上游5′-AGCAGCATTGTACAGGG-3′,下游5′-GTGCAGGGTCCGAGGT-3′;内参U6(106 bp)上游5′-TGCGGGTGCTCGCTTCGGCAGC-3′,下游5′-GTGCAGGGTCCGAGGT-3′。
1.2.6 Western blot检测SHC3蛋白RIPA法提取各组PC12细胞总蛋白。每泳道加入40 μg蛋白质样品用SDS-PAGE凝胶分离,并湿转到聚偏二氟乙烯膜(PVDF)上。室温下,将膜放入孵育盒用5%脱脂牛奶封闭2 h。用1 ∶1 000稀释的SHC3抗体、1 ∶3 000稀释的GAPDH抗体4 ℃孵育10 h。再用1 ∶3 000稀释的二抗室温孵育1 h。用增强化学发光试剂盒检测印迹,以Image J软件测得的SHC3和GAPDH灰度值比值表示SHC3蛋白表达水平。
1.2.7 双荧光素酶报告实验将含有miR-103a-3p预测结合位点的野生型(WT)SHC3-3’-非翻译区(UTR)序列及其相应的突变型(MUT)序列分别克隆到荧光素酶报告载体pmirGLO,将构建的重组载体命名为WT-SHC3、MUT-SHC3。将miR-103a-3p mimic、miR-NC分别与上述重组载体共转染PC12细胞。转染48 h后,用双荧光素酶报告检测系统测定萤火虫和肾素酶活性,以二者的比值表示相对荧光素酶活性。
1.3 统计学分析用SPSS 18.0统计软件进行数据分析,实验数据均以x±s表示。用独立样本t检验确定两组间数据差异;采用One-way ANOVA确定多组间差异,进一步两组比较采用LSD-t检验。P<0.05为差异有统计学意义。
2 结果 2.1 鸢尾苷元对PD模型损伤的影响与Con组比较,PD组PC12细胞光密度值显著降低(P<0.05),凋亡率显著升高(P<0.05);与PD组比较,PD+鸢尾苷元50、100、200 μmol/L组PC12细胞光密度值均显著升高(P<0.05),凋亡率显著降低(P<0.05),见图 1。结果表明鸢尾苷元可提高PD模型细胞活力,抑制细胞凋亡。
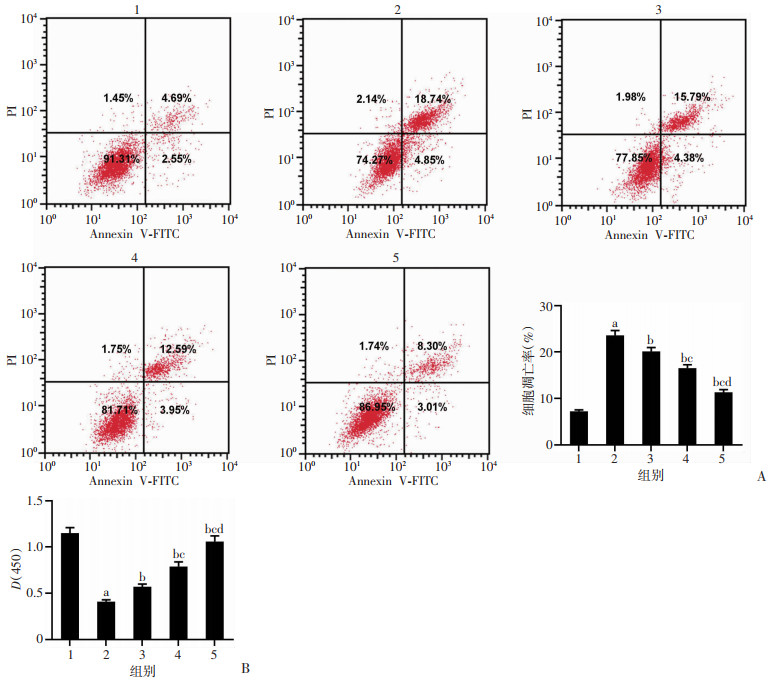
|
|
1:Con组;2:PD组;3:PD+鸢尾苷元50 μmol/L组;4:PD+鸢尾苷元100 μmol/L组;5:PD+鸢尾苷元200 μmol/L组;a:P<0.05,与Con组比较;b:P<0.05,与PD组比较;c:P<0.05,与PD+鸢尾苷元50 μmol/L组比较;d:P<0.05,与PD+鸢尾苷元100 μmol/L组比较 A:流式细胞术检测各组细胞凋亡;B:CCK-8法检测各组细胞活性 图 1 鸢尾苷元对MPP+诱导的PD模型细胞凋亡和活性的影响 |
2.2 鸢尾苷元对PD模型中SOD、MDA、LDH的影响
与Con组比较,PD组PC12细胞SOD活性显著降低(P<0.05),MDA水平、LDH释放量显著升高(P<0.05);与PD组比较,PD+鸢尾苷元50、100、200 μmol/L组PC12细胞SOD活性显著升高(P<0.05),MDA水平、LDH释放量显著降低(P<0.05),见图 2。结果表明鸢尾苷元可抑制PD模型细胞氧化应激损伤。
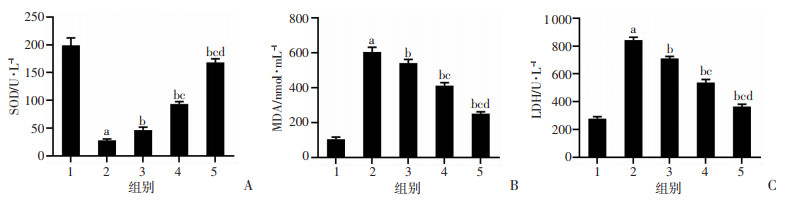
|
| 1:Con组;2:PD组;3:PD+鸢尾苷元50 μmol/L组;4:PD+鸢尾苷元100 μmol/L组;5:PD+鸢尾苷元200 μmol/L组;a:P<0.05,与Con组比较;b:P<0.05,与PD组比较;c:P<0.05,与PD+鸢尾苷元50 μmol/L组比较;d:P<0.05,与PD+鸢尾苷元100 μmol/L组比较 图 2 鸢尾苷元对PD模型中SOD(A)、MDA(B)、LDH(C)的影响 |
2.3 鸢尾苷元对PD模型中miR-103a-3p和SHC3表达的影响
与Con组比较,PD组PC12细胞miR-103a-3p表达显著升高(P<0.05),SHC3蛋白表达显著降低(P<0.05);与PD组比较,PD+鸢尾苷元50、100、200 μmol/L组PC12细胞miR-103a-3p表达显著降低(P<0.05),SHC3蛋白表达显著升高(P<0.05),见图 3。结果表明鸢尾苷元抑制PD模型细胞中miR-103a-3p表达,促进SHC3蛋白表达。
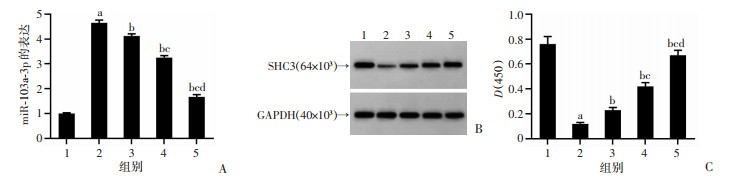
|
|
1:Con组;2:PD组;3:PD+鸢尾苷元50 μmol/L组;4:PD+鸢尾苷元100 μmol/L组;5:PD+鸢尾苷元200 μmol/L组;a:P<0.05,与Con组比较;b:P<0.05,与PD组比较;c:P<0.05,与PD+鸢尾苷元50 μmol/L组比较;d:P<0.05,与PD+鸢尾苷元100 μmol/L组比较 A:鸢尾苷元抑制MPP+诱导的PD模型中miR-103a-3p表达;B、C:鸢尾苷元促进MPP+诱导的PD模型中SHC3蛋白的表达 图 3 鸢尾苷元对MPP+诱导的PD模型中miR-103a-3p和SHC3蛋白表达的影响 |
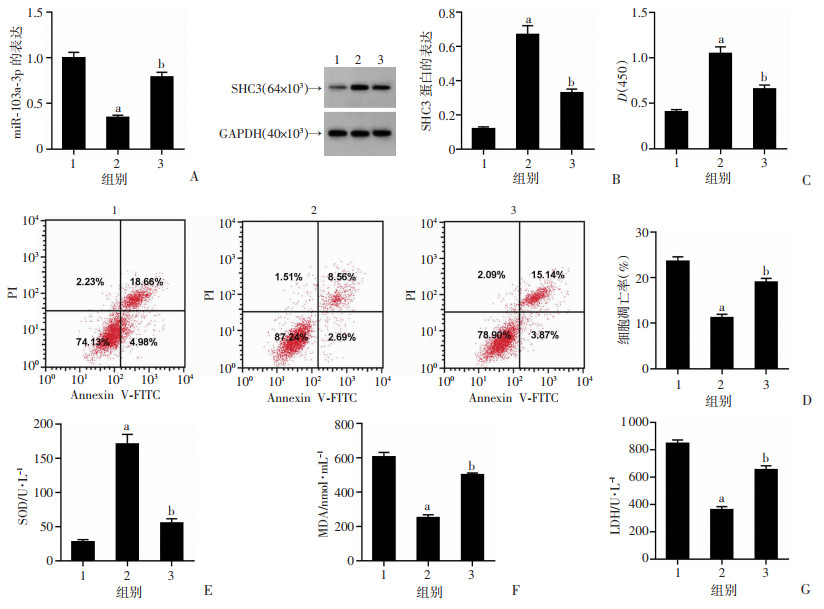
2.4 抑制miR-103a-3p对PD模型损伤及SOD、MDA、LDH的影响
与PD+anti-miR-NC组比较,PD+miR-103a-3p Inhibitor组PC12细胞miR-103a-3p表达、凋亡率、MDA水平、LDH释放量显著降低(P<0.05),SHC3蛋白表达、细胞活力、SOD活性显著升高(P<0.05),见图 4。结果表明抑制miR-103a-3p表达可抑制PD模型细胞凋亡,减轻氧化应激损伤。
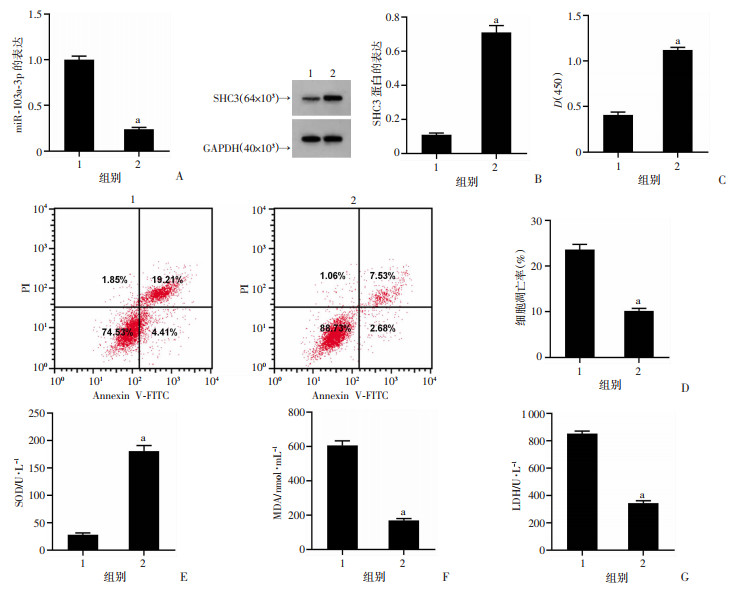
|
|
1:PD+anti-miR-NC组;2:PD+miR-103a-3p Inhibitor组;a:P<0.05,与PD+anti-miR-NC组比较 A:miR-103a-3p表达;B:SHC3蛋白表达;C:抑制miR-103a-3p促进PD模型细胞活力;D:抑制miR-103a-3p抑制PD模型细胞凋亡率;E:SOD活性;F:MDA水平;G:LDH释放量 图 4 抑制miR-103a-3p对PD模型细胞活性和凋亡及SOD、MDA、LDH的作用 |
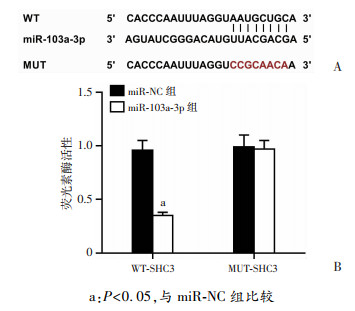
2.5 miR-103a-3p和SHC3靶向关系
Targetscan预测到miR-103a-3p和SHC3的3’-UTR区域存在互补序列,见图 5。与miR-NC和WT-SHC3共转染组比较,miR-103a-3p和WT-SHC3共转染组PC12细胞相对荧光素酶活性显著下降(P<0.05);与miR-NC和MUT-SHC3共转染组比较,miR-103a-3p和MUT-SHC3共转染组PC12细胞相对荧光素酶活性差异无统计学意义。结果表明SHC3是miR-103a-3p的直接靶点。
|
| a:P<0.05,与miR-NC组比较 图 5 miR-103a-3p和SHC3互补序列(A)及荧光素酶活性检测(B) |
2.6 过表达miR-103a-3p对鸢尾苷元处理的PD模型损伤及SOD、MDA、LDH的影响
与PD组比较,PD+鸢尾苷元组PC12细胞miR-103a-3p表达、凋亡率、MDA水平、LDH释放量显著降低(P<0.05),SHC3蛋白表达、细胞活力、SOD活性显著升高(P<0.05);与PD+鸢尾苷元组比较,PD+鸢尾苷元+miR-103a-3p mimic组PC12细胞miR-103a-3p表达水平、凋亡率、MDA水平、LDH释放量显著升高(P<0.05),SHC3蛋白表达、细胞活力、SOD活性显著降低(P<0.05),见图 6。结果表明过表达miR-103a-3p可减轻鸢尾苷元对PD模型细胞损伤的保护作用。
|
|
1:PD组;2:PD+鸢尾苷元组;3:PD+鸢尾苷元+miR-103a-3p mimic组;a:P<0.05,与PD组比较;b:P<0.05,与PD+鸢尾苷元组比较 A:miR-103a-3p表达;B:SHC3蛋白表达;C:细胞活力;D:细胞凋亡情况;E:SOD活性;F:MDA水平;G:LDH释放量 图 6 过表达miR-103a-3p可逆转鸢尾苷元对PD模型细胞活性和凋亡及SOD、MDA、LDH的作用 |
3 讨论
研究者们尽管在PD动物和细胞模型上进行了大量临床前研究,但目前仍缺乏对PD有效的治疗药物。鸢尾苷元被认为是一种潜在的神经保护剂,其通过抗氧化应激来预防神经退行性变[10]。本研究证实鸢尾苷元减轻了MPP+诱导的PC12细胞活力下降和凋亡增加,表明鸢尾苷元可抑制MPP+诱导的细胞损伤。NOH等[11]证实鸢尾苷元预处理可明显减弱紫外线B诱导的细胞凋亡,对人角质形成细胞具有抗皮肤损伤作用。YAO等[12]的研究表明鸢尾苷元可显著提高糖氧剥夺/复氧诱导的HT-22细胞凋亡,促进细胞存活。SOD是重要的细胞内抗氧化酶,其可抵抗活性氧诱导的氧化应激,MDA作为氧化应激的标志物,在不饱和脂质氧化降解过程中SOD可抑制活性氧诱导的MDA产生,进而抑制神经毒性[13]。本研究发现MPP+诱导可提高MDA水平,降低SOD活性,增加LDH释放量,但鸢尾苷元预处理可阻止上述反应,表明鸢尾苷元可抑制MPP+诱导的氧化应激损伤。与本研究结果类似,鸢尾苷元通过提高SOD活性,降低LDH漏量、MDA水平,抑制H2O2诱导的人脐静脉细胞氧化损伤[14]。MA等[15]证实鸢尾苷元通过提高肺组织中抗氧化酶活性进而抑制脂多糖诱导的急性肺损伤。此外,本研究发现鸢尾苷元预处理能够下调MPP+诱导的PC12细胞中miR-103a-3p表达水平,下调SHC3蛋白表达水平,这提示鸢尾苷元的保护作用可能与调控miR-103a-3p和SHC3表达有关。
目前,关于miR-103a-3p在细胞损伤模型中作用报道不一。LU等[16]发现循环miR-103a-3p通过蔗糖非酵解型蛋白激酶2(SNRK)/核因子κB(NF-κB)/p65调节轴参与血管紧张素Ⅱ诱导的肾脏炎症和纤维化。ZHOU等[17]证实脓毒症患者和小鼠血清中miR-103a-3p表达上调,干预miR-103a-3p表达可抑制细胞凋亡、炎症反应和氧化反应,改善脂多糖诱导的脓毒性肝损伤。然而,miR-103a-3p通过靶向高迁移率族蛋白1(HMGB1)则抑制脓毒症诱导的肝损伤和糖氧剥夺诱导的脑缺血再灌注损伤,这种差异可能与miR-103a-3p细胞特异表达模式有关[18-19]。本研究结果表明抑制miR-103a-3p表达可提高MPP+诱导的PC12细胞活力和SOD活性,降低细胞凋亡率、LDH漏量和MDA水平,这与ZHOU等[4]报道抑制miR-103a-3p表达可抑制PD进展基本吻合。SHC3肝癌[20]、脑胶质瘤[21]中被证实异位过表达发挥促癌作用。本研究表明过表达SHC3可提高MPP+诱导的PC12细胞活力和SOD活性,降低细胞凋亡率、LDH漏量和MDA水平,与齐献忠等[9]报道的PD保护作用一致。由于抑制miR-103a-3p表达、过表达SHC3与鸢尾苷元对PD模型细胞的保护作用一致,且miR-103a-3p靶向miR-103a-3p并负调控其表达,本研究推测鸢尾苷元可能通过调控miR-103a-3p/SHC3轴发挥功能。回复实验表明,过表达miR-103a-3p显著减弱鸢尾苷元对MPP+诱导的PC12细胞活力下降、凋亡以及氧化应激损伤的保护作用,这间接证实鸢尾苷元通过下调miR-103a-3p/SHC3轴保护PD模型细胞损伤。
综上所述,本研究证实鸢尾苷元可保护PD模型细胞凋亡和氧化损伤,其机制与抑制miR-103a-3p/SHC3通路有关。这些结果为鸢尾苷元、miR-103a-3p/SHC3轴在PD中的作用提供了新的认识,为PD治疗提供了潜在途径。
| [1] |
ELLIS J M, FELL M J. Current approaches to the treatment of Parkinson's disease[J]. Bioorg Med Chem Lett, 2017, 27(18): 4247-4255. |
| [2] |
FERRANTE M, CONTI G O. Environment and neurodegene-rative diseases: an update on miRNA role[J]. Microrna, 2017, 6(3): 157-165. |
| [3] |
RUPAIMOOLE R, SLACK F J. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases[J]. Nat Rev Drug Discov, 2017, 16(3): 203-222. |
| [4] |
ZHOU J J, ZHAO Y, LI Z L, et al. MiR-103a-3p regulates mitophagy in Parkinson's disease through Parkin/Ambra1 signaling[J]. Pharmacol Res, 2020, 160: 105197. |
| [5] |
ULIVIERI C, SAVINO M T, LUCCARINI I, et al. The adap- tor protein rai/ShcC promotes astrocyte-dependent inflammation during experimental autoimmune encephalomyelitis[J]. J Immunol, 2016, 197(2): 480-490. |
| [6] |
GONG J, ZHANG L, ZHANG Q, et al. Lentiviral vector-mediated SHC3 silencing exacerbates oxidative stress injury in nigral dopamine neurons by regulating the PI3K-AKT-FoxO signaling pathway in rats with Parkinson's disease[J]. Cell Physiol Biochem, 2018, 49(3): 971-984. |
| [7] |
王金凤, 薄华本, 孟祥颖, 等. 鸢尾苷元对急性心肌梗死小鼠心肌的保护作用[J]. 中国新药与临床杂志, 2010, 29(2): 99-103. WANG J F, BO H B, MENG X Y, et al. Cardioprotective function of tectorigenin in acute myocardial infarction in mice[J]. Chin J New Drugs Clin Remedies, 2010, 29(2): 99-103. |
| [8] |
ZHANG L, ZHAO Y, FAN L, et al. Tectorigenin protects against experimental fulminant hepatic failure by regulating the TLR4/mitogen-activated protein kinase and TLR4/nuclear factor-κB pathways and autophagy[J]. Phytother Res, 2019, 33(4): 1055-1064. |
| [9] |
齐献忠, 邢英瀛, 秦慧兵. 过表达SHC3激活AKT/Nrf2/GSH通路保护帕金森病氧化应激损伤[J]. 中国老年学杂志, 2019, 39(17): 4333-4337. QI X Z, XING Y Y, QIN H B. Overexpression of SHC3 activates AKT/Nrf2/GSH pathway to protect Parkinson's disease from oxidative stress injury[J]. Chin J Gerontol, 2019, 39(17): 4333-4337. |
| [10] |
PARK J S, JUNG J S, JEONG Y H, et al. Antioxidant mechanism of isoflavone metabolites in hydrogen peroxide-stimulated rat primary astrocytes: critical role of hemeoxygenase-1 and NQO1 expression[J]. J Neurochem, 2011, 119(5): 909-919. |
| [11] |
NOH D, CHOI J G, HUH E, et al. Tectorigenin, a flavonoid-based compound of leopard lily rhizome, attenuates UV-B-induced apoptosis and collagen degradation by inhibiting oxidative stress in human keratinocytes[J]. Nutrients, 2018, 10(12): E1998. |
| [12] |
YAO L, YANG M, ZHANG J, et al. Tectorigenin attenuates the OGD/R-induced HT-22 cell damage through regulation of the PI3K/AKT and the PPARγ/NF-κB pathways[J]. Hum Exp Toxicol, 2021, 40(8): 1320-1331. |
| [13] |
YANG Y, KONG F C, DING Q Q, et al. Bruceine D elevates Nrf2 activation to restrain Parkinson's disease in mice through suppressing oxidative stress and inflammatory response[J]. Biochem Biophys Res Commun, 2020, 526(4): 1013-1020. |
| [14] |
CHEN X, ZHANG W, SUN L, et al. Tectorigenin protect HUVECs from H2O2-induced oxidative stress injury by regulating PI3K/Akt pathway[J]. Tissue Cell, 2021, 68: 101475. |
| [15] |
MA C H, LIU J P, QU R, et al. Tectorigenin inhibits the inflammation of LPS-induced acute lung injury in mice[J]. Chin J Nat Med, 2014, 12(11): 841-846. |
| [16] |
LU Q L, MA Z J, DING Y, et al. Circulating miR-103a-3p contributes to angiotensin Ⅱ-induced renal inflammation and fibrosis via a SNRK/NF-κB/p65 regulatory axis[J]. Nat Commun, 2019, 10(1): 2145. |
| [17] |
ZHOU Y P, XIA Q. Inhibition of miR-103a-3p suppresses lipopolysaccharide-induced sepsis and liver injury by regulating FBXW7 expression[J]. Cell Biol Int, 2020, 44(9): 1798-1810. |
| [18] |
LI J S, HE W L, WANG Y, et al. MiR-103a-3p alleviates oxidative stress, apoptosis, and immune disorder in oxygen-glucose deprivation-treated BV2 microglial cells and rats with cerebral ischemia-reperfusion injury by targeting high mobility group box 1[J]. Ann Transl Med, 2020, 8(20): 1296. |
| [19] |
CHEN L F, LU Q, DENG F M, et al. MiR-103a-3p could attenuate sepsis-induced liver injury by targeting HMGB1[J]. Inflammation, 2020, 43(6): 2075-2086. |
| [20] |
LIU Y, ZHANG X R, YANG B C, et al. Demethylation-induced overexpression of Shc3 drives c-raf-independent activation of MEK/ERK in HCC[J]. Cancer Res, 2018, 78(9): 2219-2232. |
| [21] |
AZZALIN A, MORETTI E, ARBUSTINI E, et al. Cell density modulates SHC3 expression and survival of human glioblastoma cells through Fak activation[J]. J Neurooncol, 2014, 120(2): 245-256. |




