新辅助化疗(neoadjuvant chemotherapy,NAC)最初主要用于局部中晚期乳腺癌患者,目的是减少肿瘤负荷,增加手术机会[1]。如今,新辅助化疗已被纳入指南,运用更加广泛[2]。然而,对于存在化疗耐受的患者,选择NAC会带来诸多不利影响,例如耽误患者病情、浪费医疗资源、产生医患矛盾等。既往研究表明雌激素受体(estrogen receptor,ER)阳性型乳腺癌较阴性型对化疗敏感性差[3-4]。ER阳性型乳腺癌患者NAC化疗获益仍有争议,对此,本研究探讨影响ER阳性型乳腺癌患者NAC疗效的因素,并构建此类患者NAC疗效预测模型,为临床工作提供一定参考。
1 资料与方法 1.1 研究对象选取2015-2018年本科住院诊治的321例乳腺癌患者。诊断标准为乳腺肿块活组织粗针穿刺病理检查确诊为浸润性乳腺癌。研究对象均为女性,年龄23~79岁,平均49.83岁。纳入病例中TEC方案(表柔比星75 mg/m2 +环磷酰胺500 mg/m2 +多西他赛75 mg/m2) 291例;TC方案(环磷酰胺500 mg/m2 +多西他赛75 mg/m2)13例;EC方案(表柔比星90 mg/m2 +环磷酰胺600 mg/m2,每周3次)17例。
收集患者临床病理资料:年龄、体质量指数(body mass index,BMI)、月经状态、肿瘤生长部位、肿块大小、肿块穿刺免疫组化[雌激素受体(estrogen receptor,ER)、孕激素受体(progesterone receptor,PR)、Her-2、p53、Ki-67表达情况]、肿块血供情况、外周血中性粒细胞数与淋巴细胞数比值(neutrophil-to-lymphocyte ratio,NLR)、外周血血小板数与淋巴细胞数比值(platelet-to-lymphocyte ratio,PLR)、纤维蛋白原(fibrinogen,FBG)、临床评估腋窝淋巴结状态(clinical node stage,cN)、化疗方案。本课题已通过重庆医科大学附属第一医院伦理委员会审批(2020-202)。
1.2 纳入标准与排除标准纳入标准:①患者基本信息及临床病理资料完整;②免疫组化结果显示ER阳性表达;③术前化疗4个周期;④入院前未接受任何抗肿瘤相关治疗;⑤患者新辅助化疗后均行乳腺手术。排除标准:①合并其他部位的恶性肿瘤;②双侧乳腺癌;③入院即发生远处转移。
1.3 结果判定及分组标准临床评估腋窝淋巴结状态根据彩超以及临床查体综合判断。彩色多普勒血流信号按Adler半定量法分为4级:0~1级血供较差,2~3级血供较好[5-6]。病理诊断阳性细胞数≥1%被定义为ER、PR阳性;免疫组化CerbB-2强阳性(+++)或中阳性(++),进一步Fish检测提示基因扩增被定义为Her-2阳性(病理诊断均由重庆医科大学两位以上病理科医师独立完成)。病理完全缓解(pathologic complete response,pCR)定义为化疗后原发区域及区域淋巴结均无浸润性癌残留或仅残留原位癌。
分组标准:新辅助化疗后进行疗效评估,临床评估参照实体肿瘤的疗效评价标准(RECIST 1.1版)[5-6],临床评估完全缓解(complete response,CR)或部分缓解(partial response,PR)的患者作为有效组,而疾病稳定(stable disease,SD)和疾病进展(progressive disease,PD)作为无效组。
1.4 统计学分析采用SPSS 25.0软件及R语言4.0.0版本进行统计学分析。计量资料以x±s表示,t检验用于分析正态分布变量,非秩和检验用于非正态分布变量。计数资料以率或构成比表示,χ2检验(或Fisher确切概率法)用于组间比较。采用受试者工作特征(receiver operating characteristic,ROC)曲线计算最大Youden指数,并据此得出PLR、NLR及FBG的截断值[7]。采用二元Logistic进行单因素分析,将有意义的因素纳入多因素Logistic回归分析。根据上述方法得出的独立预测因素运用R语言构建化疗疗效预测模型,并绘制ROC曲线及校正曲线判断预测能力。
2 结果 2.1 患者的临床病理特征比较患者总体人数321人,NAC后疗效评估有效组242人(有效率为75.39%),pCR组49人(pCR率为15.26%)。BMI、肿块生长位置(左、右乳)、PLR、NLR、FBG、PR表达情况、Her-2表达情况、p53表达情况在两组间差异均无统计学意义(P>0.05);而年龄、月经状态、肿瘤最大径、cN、肿块血供情况、ER表达情况、Ki-67表达情况及化疗方案在两组间差异均有统计学意义(P < 0.05),详见表 1。
| 临床病理项目 | 总体(n=321) | 有效组(n=242) | 无效组(n=79) | χ2/t值 | P值 |
| 年龄/岁 | 49.83±9.66 | 48.91±9.47 | 52.67±9.74 | 3.048 | 0.002 |
| BMI/kg·m-2 | 24.12±3.29 | 24.06±3.28 | 24.32±3.33 | 0.606 | 0.716 |
| 肿块位置 | |||||
| 左乳 | 166(51.71) | 128(77.11) | 38(22.89) | 0.548 | 0.459 |
| 右乳 | 155(48.29) | 114(73.55) | 41(26.45) | ||
| 月经状态 | |||||
| 绝经前 | 186(57.94) | 148(79.57) | 38(20.43) | 4.166 | 0.041 |
| 已绝经 | 135(42.06) | 94(69.63) | 41(30.37) | ||
| 肿瘤最大径/cm | |||||
| <2 | 49(15.26) | 32(65.31) | 17(34.69) | 3.368 | 0.186 |
| 2~5 | 233(72.59) | 181(77.68) | 52(22.32) | ||
| >5 | 39(12.15) | 29(74.36) | 10(25.64) | ||
| 淋巴结状态 | |||||
| cN0 | 155(48.29) | 105(67.74) | 50(32.26) | 9.448 | 0.002 |
| cNX | 166(51.71) | 137(82.53) | 29(17.47) | ||
| PLR | |||||
| ≤102.84 | 226(70.40) | 174(76.99) | 52(23.01) | 1.056 | 0.304 |
| >102.84 | 95(29.60) | 68(71.58) | 27(28.42) | ||
| NLR | |||||
| ≤2.34 | 214(66.67) | 160(74.77) | 54(25.23) | 0.134 | 0.714 |
| >2.34 | 107(33.33) | 82(76.64) | 25(23.36) | ||
| FBG | |||||
| ≤2.60 | 67(20.87) | 49(73.13) | 18(26.87) | 0.232 | 0.630 |
| >2.60 | 254(79.13) | 193(75.98) | 61(24.02) | ||
| 血供情况 | |||||
| 较差 | 107(33.33) | 62(57.94) | 45(42.06) | 26.327 | <0.001 |
| 良好 | 214(66.67) | 180(84.11) | 34(15.89) | ||
| ER表达(%) | |||||
| <10 | 31(9.66) | 30(96.77) | 1(3.23) | 10.993 | 0.004 |
| 10~60 | 118(36.76) | 92(77.97) | 26(22.03) | ||
| >60 | 172(53.58) | 120(69.77) | 52(30.23) | ||
| PR表达(%) | |||||
| <10 | 119(37.07) | 89(74.79) | 30(25.21) | 0.370 | 0.848 |
| ≥10 | 202(62.93) | 153(75.74) | 49(24.26) | ||
| Ki-67表达(%) | |||||
| <20 | 114(35.51) | 68(59.65) | 46(40.35) | 22.871 | <0.001 |
| 20~50 | 187(58.26) | 157(83.96) | 30(16.04) | ||
| >50 | 20(6.23) | 17(85.00) | 3(15.00) | ||
| Her-2 | |||||
| 阳性 | 112(34.89) | 90(80.36) | 22(19.64) | 2.288 | 0.130 |
| 阴性 | 209(65.11) | 152(72.73) | 57(27.27) | ||
| p53 | |||||
| 阳性 | 235(73.21) | 173(73.62) | 62(26.38) | 1.485 | 0.223 |
| 阴性 | 86(26.79) | 69(80.23) | 17(19.77) | ||
| 化疗方案 | |||||
| TEC(TAC) | 291(90.65) | 226(77.66) | 65(22.34) | 7.672 | 0.022 |
| EC(AC) | 17(5.30) | 9(52.94) | 8(47.06) | ||
| TC | 13(4.05) | 7(53.85) | 6(46.15) | ||
2.2 对影响ER阳性型乳腺癌NAC疗效因素Logistic回归分析
Logistic回归单因素分析结果显示ER阳性型乳腺癌NAC是否有效,与患者年龄、月经状态、cN、肿块血供情况、肿瘤最大径、ER表达情况、Ki-67表达情况及不同化疗方案有关(P < 0.05);而与BMI、肿块生长位置(左、右乳)、PLR、NLR、FBG、PR表达情况、Her-2表达情况、p53表达情况无关(P>0.05)。对单因素分析有意义的因素进行多因素分析,结果显示:患者年龄、月经状态、不同化疗方案、肿瘤最大径与NAC疗效无关(P>0.05),而cN、肿块血供情况、ER表达情况、Ki-67表达情况与ER阳性型乳腺癌NAC疗效有关,是影响ER阳性型乳腺癌NAC有效性的独立影响因素(P < 0.05,表 2)。化疗前临床评估腋窝淋巴结怀疑有癌转移、乳腺肿块血供情况较好、ER低表达、Ki-67高表达都是ER阳性型乳腺癌患者NAC有效的预测因素。
| 预测因素 | 单因素分析 | 多因素分析 | |||||||
| β | P | OR | 95%CI | β | P | OR | 95%CI | ||
| 年龄/岁 | -0.042 | 0.003 | 0.959 | 0.933~0.986 | -0.026 | 0.289 | 0.974 | 0.929~1.022 | |
| 肿块位置(左侧vs右侧) | 0.192 | 0.460 | 1.211 | 0.729~2.014 | |||||
| 月经状态(绝经前vs绝经后) | 0.530 | 0.042 | 1.699 | 1.019~2.833 | 0.091 | 0.840 | 1095 | 0.452~2.655 | |
| BMI | 0.007 | 0.556 | 1.007 | 0.985~1.029 | |||||
| 淋巴结状态(cNX vs cN0) | 0.811 | 0.002 | 2.250 | 1.333~3.797 | 0.648 | 0.034 | 1.911 | 1.050~3.478 | |
| PLR | -0.001 | 0.661 | 0.999 | 0.995~1.003 | |||||
| NLR | 0.009 | 0.932 | 1.009 | 0.822~1.238 | |||||
| FBG | -0.363 | 0.052 | 0.695 | 0.482~1.004 | |||||
| 血流 | 1.346 | <0.001 | 3.843 | 2.260~6.533 | 1.384 | <0.001 | 3.992 | 2.218~7.184 | |
| ER表达(%) | -0.016 | 0.002 | 0.984 | 0.974~0.994 | -0.016 | 0.010 | 0.984 | 0.972~0.996 | |
| PR表达(%) | -0.004 | 0.372 | 0.996 | 0.989~1.004 | |||||
| Ki-67表达(%) | 0.052 | <0.001 | 1.053 | 1.030~1.077 | 0.045 | <0.001 | 1.046 | 1.022~1.070 | |
| Her-2(阳性vs阴性) | 0.428 | 0.132 | 1.534 | 0.879~2.677 | |||||
| p53(阳性vs阴性) | -0.375 | 0.225 | 0.687 | 0.375~1.259 | |||||
| 化疗方案 | |||||||||
| TEC | 1 | 1 | |||||||
| EC | -1.128 | 0.026 | 0.324 | 0.120~0.872 | -0.947 | 0.108 | 0.388 | 0.122~1.230 | |
| TC | -1.092 | 0.057 | 0.336 | 0.109~1.033 | -0.667 | 0.331 | 0.513 | 0.134~1.970 | |
| 肿瘤最大径/mm | |||||||||
| <2 | 1 | ||||||||
| 2~5 | 0.615 | 0.070 | 1.849 | 0.952~3.593 | |||||
| >5 | 0.432 | 0.362 | 1.541 | 0.609~3.900 | |||||
2.3 对影响ER阳性型乳腺癌NAC后能否达pCR的Logistic回归分析
Logistic回归单因素分析结果显示ER阳性型乳腺癌NAC后能否达pCR,与肿瘤最大径、肿块血供情况、ER表达情况、PR表达情况、Her-2表达情况、Ki-67表达情况及不同化疗方案选择有关(P < 0.05),而与患者年龄、月经状态、BMI、肿块生长位置(左、右乳)、cN、PLR、NLR、FBG及p53表达情况无关(P>0.05)。将单因素分析有意义的因素进行多因素分析,结果显示PR表达情况、Her-2表达情况、不同化疗方案与患者NAC后pCR率无关(P>0.05),而与肿瘤最大径、肿块血供情况、ER表达情况、Ki-67表达情况有关(P < 0.05,表 3)。
| 预测因素 | 单因素分析 | 多因素分析 | |||||||
| β | P | OR | 95%CI | β | P | OR | 95%CI | ||
| 年龄/岁 | -0.024 | 0.146 | 0.977 | 0.946~1.008 | |||||
| 肿块位置(左vs右) | -0.129 | 0.678 | 0.879 | 0.478~1.615 | |||||
| 月经状态(绝经前vs 绝经后) | 0.582 | 0.081 | 1.789 | 0.931~3.438 | |||||
| BMI指数 | 0.005 | 0.192 | 1.005 | 0.997~1.013 | |||||
| 淋巴结状态(cNX vs cN0) | 0.161 | 0.606 | 1.174 | 0.637~2.164 | |||||
| PLR | 0.002 | 0.233 | 1.002 | 0.998~1.007 | |||||
| NLR | -0.054 | 0.688 | 0.947 | 0.727~1.234 | |||||
| FBG | -0.157 | 0.503 | 0.855 | 0.541~1.352 | |||||
| 血流 | 0.918 | 0.019 | 2.503 | 1.166~5.376 | 1.028 | 0.018 | 2.794 | 1.189~6.567 | |
| ER表达(%) | -0.033 | <0.001 | 0.968 | 0.957~0.979 | -0.027 | <0.001 | 0.974 | 0.961~0.986 | |
| PR表达(%) | -0.017 | 0.003 | 0.983 | 0.972~0.994 | -0.004 | 0.588 | 0.996 | 0.984~1.009 | |
| Ki-67表达(%) | 0.023 | 0.008 | 1.024 | 1.006~1.041 | 0.027 | 0.008 | 1.027 | 1.007~1.048 | |
| Her-2(阳性vs阴性) | 0.993 | 0.002 | 2.700 | 1.455~5.012 | 0.608 | 0.095 | 1.837 | 0.899~3.756 | |
| p53(阳性vs阴性) | 0.016 | 0.964 | 1.016 | 0.510~2.022 | |||||
| 化疗方案 | |||||||||
| TEC | 1 | ||||||||
| EC | -1.074 | 0.303 | 0.342 | 0.044~2.641 | |||||
| TC | -0.495 | 0.466 | 1.640 | 0.434~6.193 | |||||
| 肿瘤最大径/mm | |||||||||
| <2 | 1 | 1 | |||||||
| 2~5 | -1.188 | 0.001 | 0.305 | 0.150~0.620 | -1.349 | 0.002 | 0.259 | 0.110~0.609 | |
| >5 | -1.761 | 0.009 | 0.009 | 0.172~0.644 | -1.978 | 0.007 | 0.138 | 0.033~0.585 | |
2.4 ER阳性型乳腺癌NAC疗效的预测模型建立
将多因素Logistic回归分析影响NAC疗效独立因素纳入,包括临床评估腋窝淋巴结状态、肿块血供情况、ER表达情况与Ki-67表达情况,并使用R软件构建nomogram图,建立ER阳性型乳腺癌患者行NAC疗效的预测模型,见图 1A。根据个体每一项临床病理特征向上投射到上方评分标尺即可得出该个体每一项的分值,将每一项分值相加得到总得分,总得分越高,个体NAC有效率越高,而总得分下方对应NAC后能有效缓解的概率预测值。同样构建ER阳性型乳腺癌患者NAC后能否达pCR的预测模型,见图 1B。根据个体每一项的分值得到总得分,总得分越高,个体NAC后pCR率越高,总得分下方对应NAC后达pCR的概率预测值。

|
| 图 1 ER阳性乳腺癌NAC有效性(A)与NAC后pCR(B)的预测模型 |
2.5 预测模型评价
图 2为ER阳性型乳腺癌患者NAC有效性预测模型的ROC曲线,曲线下面积为0.785,cut-off值所在特异度及灵敏度均较高(0.696,0.760),提示预测模型具有较好的预测能力。校正曲线中对角线为标准参考线,虚线为重复抽样后的校正曲线,其越接近参考线,说明预测准确性越好。对有效率预测的校正曲线中两条曲线重合度很高,表示预测值与实际观测值之间具有良好的一致性。同样对预测NAC后能否达pCR预测模型的也做出评价,见图 3。ROC曲线面积为0.829,cut-off值所在特异度及灵敏度均较高(0.673,0.857),提示此预测模型也有较好的预测能力,校正曲线个别值略有偏差,总体准确度仍较好。
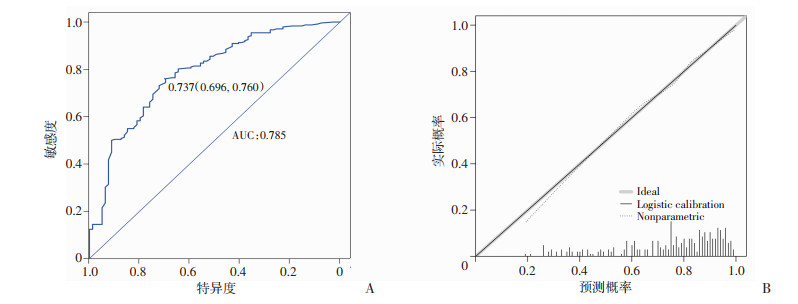
|
| A:ROC曲线;B:校正曲线 图 2 ER阳性型乳腺癌患者NAC疗效预测模型验证图 |
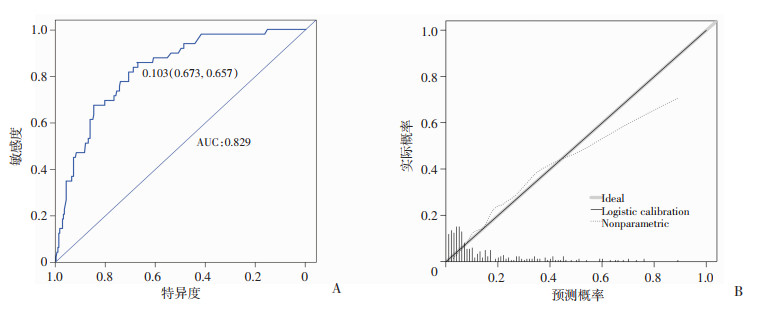
|
| A:ROC曲线;B:校正曲线 图 3 ER阳性型乳腺癌患者NAC后pCR模型的验证图 |
3 讨论
NAC已常规运用于局部中晚期乳腺癌患者,最初旨在缓解局部肿瘤负荷,为手术创造有利条件。但并不是所有类型的乳腺癌患者都能从NAC中显著获益。2000年,PEROU等[8]通过分析乳腺癌患者的8 102个人类基因的差异表达,筛选出ER-α及Her-2基因并据此将乳腺癌分为luminal型、Her-2表达型及基底样型。一项纳入8 095例乳腺癌患者的Meta分析比较了不同分型乳腺癌NAC后pCR率,结果显示不同分型的乳腺癌NAC后pCR率[luminal A型(8.3%)、luminal B型(18.7%)、Her-2阳性型(38.9%)和三阴型(31.1%)]差异有统计学意义,其中ER阳性型乳腺癌对化疗敏感性较阴性型差[9]。同样基于中国人群的研究也提示ER阳性型乳腺癌NAC效果不如ER阴性型[10]。为了从ER阳性型乳腺癌中初步筛选对化疗敏感性差,NAC疗效不佳的患者,本研究分析了影响ER阳性型乳腺癌NAC疗效的相关因素,并建立了一个客观的预测模型,对ER阳性型乳腺癌患者NAC是否获益行初步判断。
本研究中患者年龄、月经情况、BMI指数都不是NAC疗效的独立影响因素。CHOU等[11]和LEE等[12]的研究结果均提示年轻乳腺癌患者NAC后更容易达到pCR。而仉玮等[13]对luminal B型乳腺癌研究并不支持年龄是影响NAC疗效的独立因素(P=0.265)。近年来有学者提出假说,化疗主要杀伤分裂活跃的细胞,而ER阳性型乳腺癌是雌激素依赖型乳腺癌,体内游离雌激素可激活乳腺癌细胞分裂活性,有效提升化疗敏感性[14]。绝经前女性普遍体内游离雌激素较绝经后高,但目前尚未有可靠的临床证据提示月经状态与NAC疗效有显著相关性。
乳腺肿块大小和临床评估腋窝淋巴结状态对病情分期以及NAC指征判断极为重要。指南已将肿块较大(>5 cm)和腋窝淋巴结存在癌转移纳入NAC的适应证。本研究中临床评估腋窝淋巴结是否转移与NAC后能否达pCR率无相关,目前多数研究结果也显示cN与化疗后pCR率无关[15-16],但HWANG等[17]研究结论则显示cN0-1较cN2-3患者更易达pCR(OR=2.93,95%CI:1.41~6.05,P=0.004)。腋窝淋巴结是否转移与NAC能否获益的相关研究较少,本研究结果显示临床评估腋窝淋巴结有转移的患者更能从NAC获益(OR=1.911,95%CI:1.050~3.478,P=0.034)。此外,乳腺肿块越小的患者NAC后更易达pCR,这与国内外研究结论基本相同[16-17]。目前NAC主要用于局部中晚期患者,局部早期的患者往往手术后再化疗。而基于本文结果,NAC对局部早期乳腺癌患者也有一定可行性,初步评估化疗敏感性较高的局部早期乳腺癌患者,NAC可以提高保乳率或减小保乳手术的切除范围。
近年探究外周血PLR、NLR、FBG等炎症指标与乳腺癌NAC疗效以及能否达pCR的相关性的研究越来越多,而结论却是不尽相同[18-20]。本研究分析结果显示PLR、NLR、FBG与ER阳性乳腺癌NAC疗效以及化疗后pCR率均无显著相关性。笔者认为这部分炎症相关指标预测化疗疗效并不准确,感染、乳腺肿块穿刺等情况都可能引起血象改变而影响结果。恶性肿瘤血供一般较为丰富,NAC是在乳腺癌原发灶结构未被破坏时进行治疗,静脉化疗药物通过血流到达原发灶,与手术后化疗相比可以得到较好的疗效。邹淑伟等[21]通过自动乳腺容积成像(automated breast volume scanner,ABVS)联合彩超评估了72例乳腺癌患者,血供情况较好的患者化疗有效性更高。王铁芳[22]也得出同样结论。术前对患者肿块血流评估是NAC疗效的重要预测因素,而临床往往忽视其重要性,通过彩超下血流显像(color doppler flow imaging,CDFI)可了解肿瘤内血流分布特点,为NAC疗效评估提供重要参考[23]。
目前ER、PR、Her-2以及Ki-67的表达情况已成为乳腺癌常规病理指标,通过简单的免疫组化测定便可快速分型,还可以初步判断肿瘤恶性程度以及对化疗敏感的敏感性。本研究结论显示ER及Ki-67的表达情况是预测原发灶对化疗是否敏感的重要因素。化疗药物主要作用于增殖分裂活跃的细胞,而Ki-67的表达水平正好反映了细胞增殖能力,与化疗敏感性有着密切的相关性。SUETA等[24]回顾性分析了不同分型乳腺癌化疗前Ki-67对NAC疗效的预测价值,在ER表达>10%的亚组分析中,Ki-67表达水平与化疗后能否pCR有显著相关性(P=0.004 2),同时多因素分析显示Ki-67是ER阳性乳腺癌NAC后能否pCR的独立影响因素(OR=6.24,95%CI:1.40~27.7,P=0.016)。WANG等[10]分析了188例包括luminal A与luminal B型在内的ER阳性型乳腺癌患者新辅助化疗结果,总体pCR率为9.57%,结果也提示Ki-67表达水平与化疗疗效显著相关,与本研究结果相符。ER阳性型乳腺癌为雌激素依赖型乳腺癌,化疗敏感性相对ER阴性型乳腺癌较差,原因可能是这类乳腺癌异型性较低,以及存在ER介导的化疗耐药通路的激活[25]。国内外研究通常在ER阳性与ER阴性两组间比较,ER阴性NAC疗效及pCR率明显较ER阳性型好。本研究比较了不同ER表达水平的乳腺癌NAC后的有效性及pCR率,结果均呈负相关并有显著的相关性,ER表达情况是预测患者能否从NAC获益的关键因素。此外,SPRING等[26]纳入20项关于ER阳性乳腺癌新辅助内分泌治疗的研究,Meta分析得出新辅助内分泌治疗对ER阳性乳腺癌与NAC有着同样的疗效,并且不良反应更少。Her-2的表达水平与NAC的疗效在本研究并没有相关性,但是随着Her-2阳性乳腺癌新辅助化疗靶向药物的使用,Her-2阳性者NAC疗效以及化疗后pCR率会得到显著改善[27]。
本研究较全面地纳入可能与NAC有关的因素进行分析,最终构建了两个nomogram图。作为初步判断ER阳性型乳腺癌患者从NAC中的获益率以及化疗后的pCR率,两个预测模型都有较好的预测能力及准确度。医务人员可以通过这样客观的预测工具快速判断患者NAC的有效性,为患者选择个体化的治疗方式。此外,患者也可以客观了解自己能否从NAC中获益,帮助患者更直观地了解自身疾病情况。
本研究也存在不足之处,①疗效评判标准:本研究通过彩超评估患者的肿块大小,具有较大主观性;②病理结果:本研究是由患者活组织粗针穿刺病检得出结果,大部分患者无组织学分级信息,对结果有较大影响;③本研究是单中心回顾性研究,纳入患者数量较少,需更多证据来证实研究结论。
综上所述,新辅助化疗越来越多地运用于乳腺癌的治疗中,本研究建立了ER阳性型乳腺癌患者能否从NAC获益以及NAC后能否达pCR的预测模型,为此类患者选择适合的治疗方式提供一定参考,从而实现个体化、精准化治疗。
| [1] |
MIEOG J S D, VAN DER HAGE J A, VAN DE VELDE C J H. Neoadjuvant chemotherapy for operable breast cancer[J]. Br J Surg, 2007, 94(10): 1189-1200. DOI:10.1002/bjs.5894 |
| [2] |
BEVERS T B, ANDERSON B O, BONACCIO E, et al. NCCN clinical practice guidelines in oncology: breast cancer screening and diagnosis[J]. J Natl Compr Cancer Netw, 2009, 7(10): 1060. DOI:10.6004/jnccn.2009.0070 |
| [3] |
刘娜, 马天江, 支卫国. 乳腺癌分子分型在新辅助化疗疗效及预后预测中的作用[J]. 现代肿瘤医学, 2017, 25(10): 1564-1568. LIU N, MA T J, ZHI W G. The role of molecular classification of breast cancer in the efficacy and prognosis prediction of neoadjuvant chemotherapy[J]. J Mod Oncol, 2017, 25(10): 1564-1568. DOI:10.3969/j.issn.1672-4992.2017.10.013 |
| [4] |
罗益贤, 马捷, 刘永光, 等. DWI及DCE-MRI评价新辅助化疗对不同分子分型乳腺癌的疗效[J]. 医学影像学杂志, 2019, 29(6): 962-968. LUO Y X, MA J, LIU Y G, et al. The application of diffusion weighted imaging and dynamic enhanced MRI in evaluation of response of different molecular types of breast cancer to neoadjuvant chemotherapy[J]. J Med Imaging, 2019, 29(6): 962-968. |
| [5] |
ADLER D D, CARSON P L, RUBIN J M, et al. Doppler ultrasound color flow imaging in the study of breast cancer: preliminary findings[J]. Ultrasound Med Biol, 1990, 16(6): 553-559. DOI:10.1016/0301-5629(90)90020-d |
| [6] |
EISENHAUER E A, THERASSE P, BOGAERTS J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1)[J]. Eur J Cancer, 2009, 45(2): 228-247. DOI:10.1016/j.ejca.2008.10.026 |
| [7] |
FLUSS R, FARAGGI D, REISER B. Estimation of the Youden Index and its associated cutoff point[J]. Biom J, 2005, 47(4): 458-472. DOI:10.1002/bimj.200410135 |
| [8] |
PEROU C M, SØRLIE T, EISEN M B, et al. Molecular portraits of human breast tumours[J]. Nature, 2000, 406(6797): 747-752. DOI:10.1038/35021093 |
| [9] |
HOUSSAMI N, MACASKILL P, VON MINCKWITZ G, et al. Meta-analysis of the association of breast cancer subtype and pathologic complete response to neoadjuvant chemotherapy[J]. Eur J Cancer, 2012, 48(18): 3342-3354. DOI:10.1016/j.ejca.2012.05.023 |
| [10] |
WANG J, SANG D, XU B, et al. Value of breast cancer molecular subtypes and Ki-67 expression for the prediction of efficacy and prognosis of neoadjuvant chemotherapy in a Chinese population[J]. Medicine (Baltimore), 2016, 95(18): e3518. DOI:10.1097/md.0000000000003518 |
| [11] |
CHOU H H, KUO W L, YU C C, et al. Impact of age on pathological complete response and locoregional recurrence in locally advanced breast cancer after neoadjuvant chemotherapy[J]. Biomed J, 2019, 42(1): 66-74. DOI:10.1016/j.bj.2018.10.007 |
| [12] |
LEE Y J, SHIN Y I, KIM J, et al. Abstract P2-16-25: Young age breast cancer who achieve pathologic complete response after neoadjuvant chemotherapy display excellent outcome than older age breast cancers[J]. Cancer Res, 2020, 80(4 Supplement): P2-16-25. DOI:10.1158/1538-7445.sabcs19-p2-16-25 |
| [13] |
仉玮, 赖米林, 杨时鸿, 等. Luminal B型乳腺癌新辅助化疗效果与影响因素分析[J]. 广东医学, 2017, 38(22): 3426-3429, 3432. ZHANG W, LAI M L, YANG S H, et al. Neoadjuvant chemotherapy effect and influencing factors on Luminal B breast cancer[J]. Guangdong Med J, 2017, 38(22): 3426-3429, 3432. |
| [14] |
LUO Q Q, HUANG J B, WU Y T, et al. Tidal chemotherapy in premenopausal patients with hormone receptor positive breast cancer[J]. Med Hypotheses, 2017, 102: 4-7. DOI:10.1016/j.mehy.2017.03.003 |
| [15] |
CHOI H J, RYU J M, KIM I, et al. Nomogram for accurate prediction of breast and axillary pathologic response after neoadjuvant chemotherapy in node positive patients with breast cancer[J]. Ann Surg Treat Res, 2019, 96(4): 169-176. DOI:10.4174/astr.2019.96.4.169 |
| [16] |
ZHANG F, HUANG M, ZHOU H, et al. A nomogram to predict pathologic complete response of neoadjuvant chemotherapy in triple-negative breast cancer based on simple blood indicators[J]. Ann Oncol, 2019, 30: v92. DOI:10.1093/annonc/mdz240.096 |
| [17] |
HWANG H W, JUNG H, HYEON J, et al. A nomogram to predict pathologic complete response (pCR) and the value of tumor-infiltrating lymphocytes (TILs) for prediction of response to neoadjuvant chemotherapy (NAC) in breast cancer patients[J]. Breast Cancer Res Treat, 2019, 173(2): 255-266. DOI:10.1007/s10549-018-4981-x |
| [18] |
PENG Y, CHEN R, QU F, et al. Low pretreatment lymphocyte/monocyte ratio is associated with the better efficacy of neoadjuvant chemotherapy in breast cancer patients[J]. Cancer Biol Ther, 2020, 21(2): 189-196. DOI:10.1080/15384047.2019.1680057 |
| [19] |
GRAZIANO V, GRASSADONIA A, IEZZI L, et al. Combination of peripheral neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio is predictive of pathological complete response after neoadjuvant chemotherapy in breast cancer patients[J]. Breast, 2019, 44: 33-38. DOI:10.1016/j.breast.2018.12.014 |
| [20] |
CUELLO-LÓPEZ J, FIDALGO-ZAPATA A, LÓPEZ-AGUDELO L, et al. Platelet-to-lymphocyte ratio as a predictive factor of complete pathologic response to neoadjuvant chemotherapy in breast cancer[J]. PLoS ONE, 2018, 13(11): e0207224. DOI:10.1371/journal.pone.0207224 |
| [21] |
邹淑伟, 韩彦峰, 王宝华. 自动乳腺全容积成像联合彩色多普勒血流显像对乳腺癌新辅助化疗效果的评估价值[J]. 中国乡村医药, 2019, 26(10): 54-55. ZOU S W, HAN Y F, WANG B H. The value of automatic breast full-volume imaging combined with color Doppler blood flow imaging in evaluating the effect of neoadjuvant chemotherapy for breast cancer[J]. Chin J Rural Med Pharm, 2019, 26(10): 54-55. |
| [22] |
王铁芳. 彩色多普勒血流显像联合超声弹性成像技术对乳腺癌新辅助化疗疗效的诊断价值[J]. 中国乡村医药, 2018, 25(20): 48-49. WANG T F. The diagnostic value of color Doppler flow imaging combined with ultrasound elastography for the efficacy of neoadjuvant chemotherapy for breast cancer[J]. Chin J Rural Med Pharm, 2018, 25(20): 48-49. DOI:10.19542/j.cnki.1006-5180.002143 |
| [23] |
黄梅, 王树群, 冯娜娜, 等. 超声自动乳腺全容积成像联合彩色多普勒血流显像对乳腺癌新辅助化疗疗效评价的意义[J]. 中国肿瘤临床与康复, 2017, 24(5): 570-574. HUANG M, WANG S Q, FENG N N, et al. Significance of ABVS combined with CDFI in the evaluation of efficacy of neoadjuvant chemotherapy for breast cancer[J]. Chin J Clin Oncol Rehabilit, 2017, 24(5): 570-574. |
| [24] |
SUETA A, YAMAMOTO Y, HAYASHI M, et al. Clinical significance of pretherapeutic Ki-67 as a predictive parameter for response to neoadjuvant chemotherapy in breast cancer: is it equally useful across tumor subtypes?[J]. Surgery, 2014, 155(5): 927-935. DOI:10.1016/j.surg.2014.01.009 |
| [25] |
孔琳娜. Estrogen Receptor, claudin6的表达与Luminal A型乳腺癌对蒽环类药物敏感性的相关研究[D].沈阳: 中国医科大学, 2018. KONG L N. The relationship between the expression of ER and claudin6 and the sensitivity of luminal A breast cancer to anthracycline[D]. Shenyang: China Medical University, 2018. |
| [26] |
SPRING L M, GUPTA A, REYNOLDS K L, et al. Neoadjuvant endocrine therapy for estrogen receptor-positive breast cancer: A systematic review and meta-analysis[J]. JAMA Oncol, 2016, 2(11): 1477-1486. DOI:10.1001/jamaoncol.2016.1897 |
| [27] |
HURVITZ S A, MARTIN M, SYMMANS W F, et al. Neoadjuvant trastuzumab, pertuzumab, and chemotherapy versus trastuzumab emtansine plus pertuzumab in patients with HER2-positive breast cancer (KRISTINE): a randomised, open-label, multicentre, phase 3 trial[J]. Lancet Oncol, 2018, 19(1): 115-126. DOI:10.1016/s1470-2045(17)30716-7 |



