2. 400038 重庆,陆军军医大学病理生理学教研室
2. Department of Pathophysiology, Army Medical University (Third Military Medical University), Chongqing, 400038, China
系统性红斑狼疮(systemic lupus erythematosus, SLE)是一种自身免疫性疾病,患者体内有多种自身抗体沉淀形成免疫复合物,可造成慢性炎症和多器官功能损伤,此病多见于育龄期女性[1-2]。一项全球性研究显示SLE患病率为每年9~241/10万,目前SLE的发病机制仍不清楚,研究报道SLE发病与遗传和环境因素共同作用有关[3]。环状RNA (circular RNA,circRNA)是一种具有闭合环状结构的内源性非编码RNA,在基因的表达调控方面发挥着重要作用[4]。与线性RNA相比,circRNA不仅具有更丰富的表达量,而且具有更高的稳定性[5-6]。circRNA在多种肿瘤、神经系统疾病、自身免疫性疾病中表达失调,广泛的参与各类疾病的发生、发展[7-9]。目前,已有研究表明circRNA参与SLE炎性调节过程中的基因表达[10-12]。分析circRNA与SLE早期诊断和预后的关系及其在SLE发病过程中的作用,具有重要的价值和意义[13-14]。
为探寻SLE相关circRNA分子靶标并评估其在SLE发生发展生物标志物的可能性,我们前期通过分析外周血单核细胞PBMCs(peripheral blood mononuclear cells)样本RNA-seq测序数据[15],挑选出了3个显著性差异基因[P < 0.01, fold change(差异值)>2] circADCY9,circGARS,circMCTP2为研究目标。本研究拟设计引物通过RT-qPCR验证其表达情况,对circRNA的稳定性及耐受程度进行检测,通过结合SLE临床指标关联分析评估其潜在的诊断价值及意义。
1 材料与方法 1.1 研究对象SLE组病例来自2018年3月至2019年10月在重庆西南医院就诊的患者,其中男3例,女32例;年龄7~56(37.4±12.9)岁。临床诊断符合1997年美国风湿协会修订的诊断标准[16],除外感染、肿瘤及其他结缔组织病。健康对照者(HC组)来自重庆西南医院健康体检者,其中男2例,女23例;年龄23~56(39.8±9.4)岁。两组性别年龄无显著差异。收集SLE患者临床相关指标并进行SLE疾病活动指数(systemic lupus erythematosus disease activity index,SLEDAI)评分[17]。SLEDAI评分0~18(8.5±4.9)分,其中基本无活动:10例(28.6%),轻度活动:11例(31.4%),中度活动9例(25.7%),重度活动5例(14.3%)。研究经中国人民解放军陆军军医大学第一附属医院伦理委员会批准,获得每位参与者书面知情同意。患者的临床资料见表 1。
| 临床资料 | 例数(%) |
| 皮肤表现 | 11(31.4) |
| 脱发 | 10(28.6) |
| 关节炎 | 10(28.6) |
| 蛋白尿 | 16(45.7) |
| 血尿 | 4(11.4) |
| 胸膜炎 | 8(22.8) |
| 血小板减少 | 6(17.1) |
| 白细胞减少 | 7(20.0) |
| 低补体 | 26(74.2) |
| Anti-dsDNA | 8(22.8) |
| Anti-sm | 11(31.4) |
| Anti-SSA | 15(42.8) |
| Anti-SSB | 3(8.5) |
| Anti-RO52 | 15(42.8) |
| Anti-ANA | 33(94.2) |
| Anti-RNP | 10(28.6) |
1.2 主要试剂和仪器
人单个核细胞分离液Ficoll购自天津市灏洋生物制品科技有限责任公司;总RNA提取试剂Trizol购自天根生化科技(北京)有限公司;PrimeScript RT逆转录试剂盒购自成都微克生物技术有限公司;qPCR SYBR Green购自武汉生命之美科技有限公司;基因引物均由生工生物工程(上海)股份有限公司合成;Nanodrop ND-1000紫外分光光度计为美国Nanodrop公司产品;伯乐CFX Connect PCR仪器为美国Bio-Rad公司产品。
1.3 PBMCs及总RNA提取采集受试者清晨空腹静脉血3~5 mL至EDTA抗凝管,2 h内送入实验室,Ficoll分离液分离PBMCs,Trizol试剂盒提取总RNA,通过分光光度计测量提取的RNA质量,-80 ℃保存。
1.4 RT-qPCR检测引物设计按照常规PCR引物设计原理,利用primer premier 5.0软件跨剪接位点上下游进行设计,将设计好的引物序列导入NCBI数据库(http://blast.ncbi.nlm.nih.gov/Blast.cgi)中,采用Primer-Blast工具进行引物特异性比对分析,利用PrimeScript反转录试剂盒将RNA反转录为cDNA,采用20 μL反应体系进行检测。反应体系包括:1 μL反转录产物、0. 5 μmol/L上游引物、0. 5 μmol/L下游引物、1×SYBR荧光染料试剂。PCR反应条件为:95 ℃ 5 min预变性;95 ℃ 10 s变性,60 ℃ 34 s退火延伸,读板,扩增循环40次。所有样品做3个复孔。RT-qPCR使用伯乐CFX Connect荧光定量PCR仪进行。分析待测标本的扩增熔解曲线,均为单峰,无非特异性扩增。结果以CT值显示,采用相对定量法对RT-qPCR结果进行分析,计算2-ΔCt。引物序列见表 2。
| 名称 | 引物序列(5′-3′) |
| ADCY9 | 正义:CTCCTGCTCTTGTTGGTCTGGTTC |
| 反义:CTCTGGATCTTGGTGCGGTGAAG | |
| GARS | 正义:GGCAGCACATGGAGAATGAGATGG |
| 反义:ACAGGAACGATCAGCACATCCAAC | |
| MCTP2 | 正义:GCCACAGCGATCTCGGACAAC |
| 反义:GATGCACCAGAAGAGCCAGAGAAG | |
| circADCY9 | 正义:TGAGACCAACATACACGTCAT |
| 反义:GGAGCTGAAGGTGGGACTAG | |
| circGARS | 正义:TGCCATTTGTGATGAGTGCT |
| 反义:CAACAATCTCAATCCAACCCCT | |
| circMCTP2 | 正义:TGGAGAAAGGATTAAGAAGTATT |
| 反义:GTCCTGGATGCTGCTGAC | |
| β-actin | 正义:5′-GGTGAGCTGCGAGAATAGCC-3′ |
| 反义:5′-CTCCGACCAGTGTTTGCCTT-3′ |
1.5 统计学处理
统计分析和图形采用GraphPad Prism 8.0软件进行,两组样本进行t检验,两个变量之间相关性采用Pearson相关分析。受试者工作特征(receiver operator characteristic,ROC)曲线分析评价circRNA诊断和鉴别诊断的敏感性和特异性。
2 结果 2.1 RT-qPCR检测实验组和健康对照组circADCY9,circGARS,circMCTP2表达情况结果显示circADCY9,circGARS在35例SLE患者相较25例健康人PBMCs中表达上调,circMCTP2表达下调(P均 < 0.000 1,图 1)。差异有统计学意义。
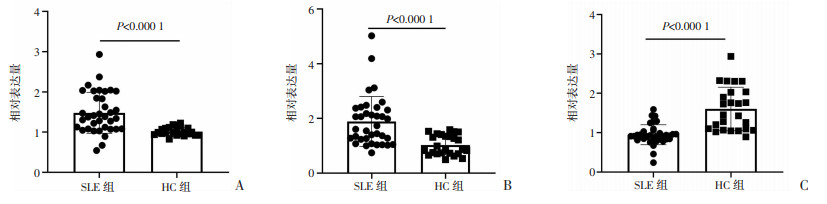
|
| 图 1 circADCY9(A)、circGARS(B)、circMCTP2(C)在实验组和对照组中的表达情况比较 |
2.2 PBMCs中circADCY9、circGARS、circMCTP2表达水平与SLE患者临床参数之间相关性分析
SLE患者circADCY9、circGARS表达水平与SLEDAI评分呈正相关(两者P值均 < 0.000 1,r分别为0.841 7和0.801 0),circMCTP2表达水平与SLEDAI评分呈负相关(P < 0.000 1,r=-0.810 2)。SLE患者circADCY9、circGARS表达水平与补体C3呈负相关(两者P值分别为 < 0.001 5和 < 0.000 5,两者r值分别为-0.509 4和-0.551 1),circMCTP2表达水平与补体C3呈正相关(P < 0.012 1,r=0.414 0,图 2、3)。而与其他临床指标(dsDNA,Anti-sm、C4、抗核抗体等)不相关。
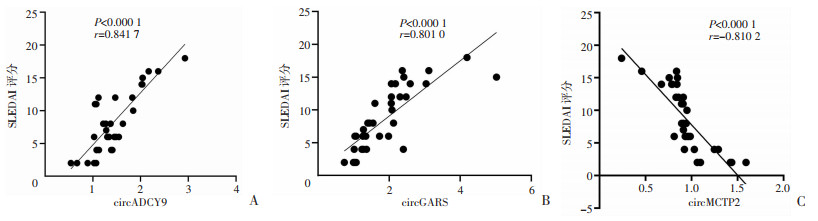
|
| 图 2 SLE患者circADCY9(A)、circGARS(B)、circMCTP2(C)表达水平与SLEDAI评分相关分析结果 |
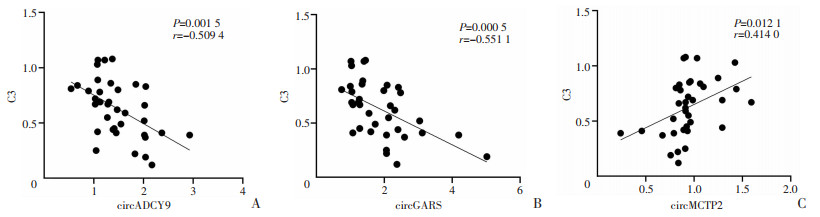
|
| 图 3 SLE患者PBMCs中circADCY9(A)、circGARS(B)、circMCTP2(C)表达水平与补体C3水平相关分析结果 |
2.3 PBMCs中3种circRNA对SLE患者诊断的价值
对SLE患者和健康对照者的PBMCs中circADCY9、circGARS、circMCTP2表达水平进行ROC曲线分析。CircADCY9的ROC曲线下AUC为0.850 0(95%CI:0.749 4-0.950 6;P < 0.0001)。CircGARS的ROC曲线下AUC为0.826 7(95%CI:0.725 0-0.928 3;P < 0.000 1)。CircMCTP2的ROC曲线下AUC为0.895 6(95%CI:0.817 8-0.973 3;P < 0.000 1)。见图 4。
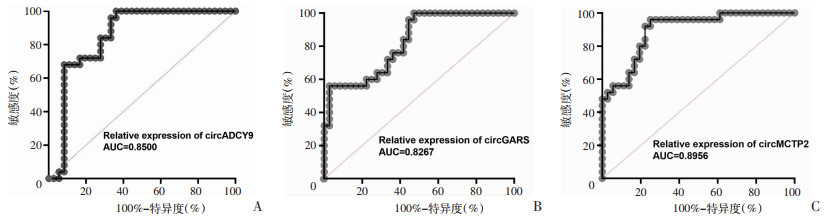
|
| 图 4 实验组和对照组PBMCs中circADCY9(A)、circGARS(B)、circMCTP2(C)的ROC曲线分析 |
2.4 RNaseR处理前后circADCY9,circGARS,circMCTP2表达差异情况比较
选取6例SLE患者和3例健康对照者PBMCs总RNA使用RNaseR处理前后circADCY9,circGARS,circMCTP2表达差异情况比较。结果显示circADCY9,circGARS在RNaseR处理前后表达上调且总体趋势一致,circMCTP2表达下调且总体趋势一致(图 5)。PCR产物电泳检测显示β-actin、circADCY9、circGARS、circMCTP2条带清晰可见(图 6)。RNaseR处理前后circADCY9及mRNA电泳检测结果提示,circADCY9在使用RNaseR处理前后表达影响不大,而β-actin及线性ADCY9条带明显变浅(图 7)。
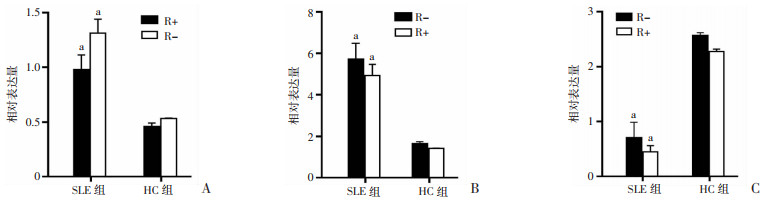
|
| a: P < 0.01, 与HC组比较 图 5 RNaseR处理前后实验组和对照组PBMCs中circADCY9(A)、circGARS(B)、circMCTP2(C)的表达情况 |
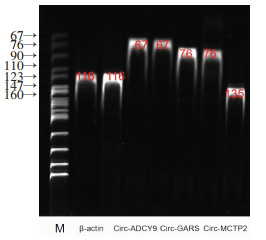
|
| 图 6 PCR检测PBMCs中circADCY9、circGARS、circMCTP2表达 |
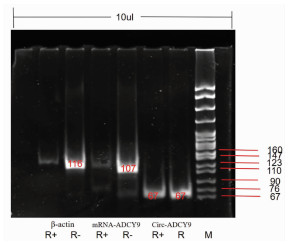
|
| 图 7 PCR检测PBMCs中使用RnaseR处理前后circADCY9、线性ADCY9表达 |
3 讨论
SLE是一种慢性全身性自身免疫性疾病,其特征是自身抗体产生,补体激活和免疫复合物沉积[18]。临床上比较常用的诊断标记物如抗核抗体(ANAs),抗双链DNA(dsDNA)抗体以及抗史密斯(Anti-Sm)抗体等对于SLE的早期筛查和诊断具有重要的意义,但是这些传统的实验室指标缺乏高特异性或者是高敏感性[19]。circRNA作为一种大量存在于哺乳动物的内源性的非编码RNA,表达丰富且具有较高的稳定性[20]。它最主要的功能是参与转录后调控,类似于一个内源性RNA或miRNA的海绵,可以竞争性的抑制RNA/miRNA的转录调控[21-22]。circRNA还可以通过抑制转录起始位点调节可变剪切或转录,调节其亲本基因的表达[23]。此外,部分circRNA分子吸附蛋白质因子与RNA结合蛋白相互作用,circRNA还可被翻译成蛋白质[24-25]。
circRNA与SLE密切相关,研究发现:hsa_circ_0045272可以充当miR-6127的海绵并对T细胞的凋亡和IL-2分泌进行调节[14];Hsa_circ_0012919在SLE患者中的下调增加甲基转移酶1的表达并降低了CD70、CD11a的表达,逆转了SLE CD4+ T细胞中CD70和CD11a的DNA甲基化不足[26];SLE患者PBMCs中存在蛋白激酶磷酸化的增强和circRNA降低,circRNA可形成不完全的RNA双链,并充当与天然免疫相关的双链RNA激活蛋白激酶的抑制剂[27];hsa_circ_0049224和has_circ_0049220可用于评估低甲基化的生物标志并参与SLE的活动[28];circIBTK在SLE中下调,并与SLEDAI评分、抗dsDNA和补体C3水平相关,circIBTK可能通过与SLE中的MIR-29b结合来调节DNA去甲基化和AKT信号通路[29];hsa_circ_0021372和hsa_circ_0075699水平与补体C3和C4表达水平有关,hsa_circ_0057762水平与SLEDAI-2K评分呈正相关[30]。以上研究提示,circRNA在SLE发生发展中发挥重要的作用,研究circRNA与SLE发病的关系,对探索SLE的生物诊断标记物及其发病机制,具有重要的意义。
本研究中circADCY9、circGARS在SLE患者中表达增高,且其表达水平与SLE患者SLEDAI评分呈正相关,与补体C3水平呈负相关,说明circADCY9、circGARS的表达水平与SLE的严重程度呈正比,患者疾病越严重,circADCY9、circGARS的表达水平越高。circMCTP2在SLE患者中低表达,其表达水平与SLE患者SLEDAI评分呈负相关,与补体C3呈正相关,说明circMCTP2的表达水平与SLE的严重程度呈反比,患者疾病越严重,circMCTP2的表达水平越低。系统性红斑狼疮活动指数(SLEDAI)具有较好的有效性和对变化的敏感性,能对SLE患者的病情严重程度进行评估[31]。补体C3的降低表明自身抗体增加,免疫激活,与SLE疾病的活动性呈负相关,在SLE的诊断中具有筛查价值[32-33]。通过评估circRNA与SLEDAI和C3的关系能够一定程度上反应circRNA与SLE严重程度和活动性关系。ROC曲线分析提示:三者曲线下面积(AUC)在0.8~0.9之间,诊断效能较好。进一步对RNaseR处理前后3种circRNA表达情况进行比较以及PCR产物跑胶验证结果提示:circRNA表达情况较为稳定,circRNA相较于mRNA对RNaseR的耐受性更好。既往的研究也证实:与线性RNA相比,circRNA分子呈封闭环状的结构,能在很大程度上能抵抗核酸外切酶的活性[34]。综上,SLE患者PBMCs中circADCY9、circGARS、circMCTP2表达明显异常,这3种circRNA表达稳定,其表达水平与SLE的严重程度具有相关性。
本研究尚存在一些不足及影响因素: ①不同SLE患者异质性较大;②本文收集的SLE病例并非均是初诊未治疗的,药物可能对其表达有影响;③SLE发病机制较复杂受限因素较多;④SLE患者的研究样本量较少;⑤没有检测治疗前后以及初诊和复诊患者之间circRNA的表达差异,无法明确其在预后监测中的疗效。在后续的研究中将进一步扩大样本量检测SLE患者PBMCs中circRNA的表达,使研究结果更具有可靠性。
| [1] |
MILLS J A. Systemic lupus erythematosus[J]. N Engl J Med, 1994, 330(26): 1871-1879. DOI:10.1056/NEJM199406303302608 |
| [2] |
LEVER E, ALVES M R, ISENBERG D A. Towards precision medicine in systemic lupus erythematosus[J]. Pharmgenomics Pers Med, 2020, 13: 39-49. DOI:10.2147/pgpm.s205079 |
| [3] |
GERGIANAKI I, BORTOLUZZI A, BERTSIAS G. Update on the epidemiology, risk factors, and disease outcomes of systemic lupus erythematosus[J]. Best Pract Res Clin Rheumatol, 2018, 32(2): 188-205. DOI:10.1016/j.berh.2018.09.004 |
| [4] |
LEDFORD H. Circular RNAs throw genetics for a loop[J]. Nature, 2013, 494(7438): 415. DOI:10.1038/494415a |
| [5] |
JECK W R, SORRENTINO J A, WANG K, et al. Circular RNAs are abundant, conserved, and associated with ALU repeats[J]. RNA, 2013, 19(2): 141-157. DOI:10.1261/rna.035667.112 |
| [6] |
MEMCZAK S, PAPAVASILEIOU P, PETERS O, et al. Identification and characterization of circular RNAs as a new class of putative biomarkers in human blood[J]. PLoS ONE, 2015, 10(10): e0141214. DOI:10.1371/journal.pone.0141214 |
| [7] |
ZHAO Y, ALEXANDROV P N, JABER V, et al. Deficiency in the ubiquitin conjugating enzyme UBE2A in Alzheimer's disease (AD) is linked to deficits in a natural circular miRNA-7 sponge (circRNA; ciRS-7)[J]. Genes (Basel), 2016, 7(12). DOI:10.3390/genes7120116.DOI:10.3390/genes7120116 |
| [8] |
LI Y W, ZHENG F X, XIAO X Y, et al. CircHIPK3 sponges miR-558 to suppressheparanase expression in bladder cancer cells[J]. EMBO Rep, 2017, 18(9): 1646-1659. DOI:10.15252/embr.201643581 |
| [9] |
LUO Q, ZENG LL, ZENG L B, et al. Expression and clinical significance of circular RNAs hsa_circ_0000175 and hsa_circ_0008410 in peripheral blood mononuclear cells from patients with rheumatoid arthritis[J]. Int J Mol Med, 2020, 45(4): 1203-1212. DOI:10.3892/ijmm.2020.4498 |
| [10] |
MEMCZAK S, JENS M, ELEFSINIOTI A, et al. Circular RNAs are a large class of animal RNAs with regulatory potency[J]. Nature, 2013, 495(7441): 333-338. DOI:10.1038/nature11928 |
| [11] |
LI L J, HUANG Q, PAN H F, et al. Circular RNAs and systemic lupus erythematosus[J]. Exp Cell Res, 2016, 346(2): 248-254. DOI:10.1016/j.yexcr.2016.07.021 |
| [12] |
CORTES R, FORNER M J. Circular RNAS: novel biomarkers of disease activity in systemic lupus erythematosus?[J]. Clin Sci (Lond), 2019, 133(9): 1049-1052. DOI:10.1042/CS20180826 |
| [13] |
CHEN LL, YANG L. Regulation of circRNA biogenesis[J]. RNA Biol, 2015, 12(4): 381-388. DOI:10.1080/15476286.2015.1020271 |
| [14] |
LI L J, ZHU Z W, ZHAO W, et al. Circular RNA expression profile and potential function of hsa_circ_0045272 in systemic lupus erythematosus[J]. Immunology, 2018, 155(1): 137-149. DOI:10.1111/imm.12940 |
| [15] |
MIAO Q, ZHONG Z, JIANG Z, et al. RNA-seq of circular RNAs identified circPTPN22 as a potential new activity indicator in systemic lupus erythematosus[J]. Lupus, 2019, 28(4): 520-528. DOI:10.1177/0961203319830493 |
| [16] |
HOCHBERG M C. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus[J]. Arthritis Rheum, 1997, 40(9): 1725. DOI:10.1002/art.1780400928 |
| [17] |
BOMBARDIER C, GLADMAN D D, UROWITZ M B, et al. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE[J]. Arthritis Rheum, 1992, 35(6): 630-640. DOI:10.1002/art.1780350606 |
| [18] |
CHOI M Y, FLOOD K, BERNATSKY S, et al. A review on SLE and malignancy[J]. Best Pract Res Clin Rheumatol, 2017, 31(3): 373-396. DOI:10.1016/j.berh.2017.09.013 |
| [19] |
ZHAO M, ZHOU Y, ZHU B C, et al. IFI44L promoter methylation as a blood biomarker for systemic lupus erythematosus[J]. Ann Rheum Dis, 2016, 75(11): 1998-2006. DOI:10.1136/annrheumdis-2015-208410 |
| [20] |
ZHANG Y, ZHANG X O, CHEN T, et al. Circular intronic long noncoding RNAs[J]. Mol Cell, 2013, 51(6): 792-806. DOI:10.1016/j.molcel.2013.08.017 |
| [21] |
HANSEN T B, JENSEN T I, CLAUSEN B H, et al. Natural RNA circles function as efficient microRNA sponges[J]. Nature, 2013, 495(7441): 384-388. DOI:10.1038/nature11993 |
| [22] |
TAULLI R, LORETELLI C, PANDOLFI PP. From pseudo-ceRNAs to circ-ceRNAs: a tale of cross-talk and competition[J]. Nat Struct Mol Biol, 2013, 20(5): 541-543. DOI:10.1038/nsmb.2580 |
| [23] |
QU S B, YANG X S, LI X L, et al. Circular RNA: a new star of noncoding RNAs[J]. Cancer Lett, 2015, 365(2): 141-148. DOI:10.1016/j.canlet.2015.06.003 |
| [24] |
WILUSZ J E, SHARP P A. Molecular biology. A circuitous route to noncoding RNA[J]. Science, 2013, 340(6131): 440-441. DOI:10.1126/science.1238522 |
| [25] |
GUO J U, AGARWAL V, GUO H L, et al. Expanded identification and characterization of mammalian circular RNAs[J]. Genome Biol, 2014, 15(7): 409. DOI:10.1186/s13059-014-0409-z |
| [26] |
ZHANG C Z, WANG X, CHEN Y, et al. The down-regulation of hsa_circ_0012919, the sponge for miR-125a-3p, contributes to DNA methylation of CD11a and CD70 in CD4+ T cells of systemic lupus erythematous[J]. Clin Sci (Lond), 2018, 132(21): 2285-2298. DOI:10.1042/CS20180403 |
| [27] |
LIU C X, LI X, NAN F, et al. Structure and degradation of circular RNAs regulate PKR activation in innate immunity[J]. Cell, 2019, 177(4): 865-880. DOI:10.1016/j.cell.2019.03.046 |
| [28] |
ZHANG C Z, HUANG J, CHEN Y, et al. Low expression and clinical value of hsa_circ_0049224 and has_circ_0049220 in systemic lupus erythematous patients[J]. Med Sci Monit, 2018, 24: 1930-1935. DOI:10.12659/msm.906507 |
| [29] |
WANG X, ZHANG C Z, WU Z W, et al. CircIBTK inhibits DNA demethylation and activation of AKT signaling pathway via miR-29b in peripheral blood mononuclear cells in systemic lupus erythematosus[J]. Arthritis Res Ther, 2018, 20(1): 1-10. DOI:10.1186/s13075-018-1618-8 |
| [30] |
LI S P, ZHANG J M, TAN X H, et al. Microarray expression profile of circular RNAs and mRNAs in children with systemic lupus erythematosus[J]. Clin Rheumatol, 2019, 38(5): 1339-1350. DOI:10.1007/s10067-018-4392-8 |
| [31] |
GRIFFITHS B, MOSCA M, GORDON C. Assessment of patients with systemic lupus erythematosus and the use of lupus disease activity indices[J]. Best Pract Res Clin Rheumatol, 2005, 19(5): 685-708. DOI:10.1016/j.berh.2005.03.010 |
| [32] |
LI W H, LI H, SONG W Q, et al. Differential diagnosis of systemic lupus erythematosus and rheumatoid arthritis with complements C3 and C4 and C-reactive protein[J]. Exp Ther Med, 2013, 6(5): 1271-1276. DOI:10.3892/etm.2013.1304 |
| [33] |
QU C H, ZHANG J, ZHANG X M, et al. Value of combined detection of anti-nuclear antibody, anti-double-stranded DNA antibody and C3, C4 complements in the clinical diagnosis of systemic lupus erythematosus[J]. Exp Ther Med, 2019, 17(2): 1390-1394. DOI:10.3892/etm.2018.7072 |
| [34] |
MENG S J, ZHOU H C, FENG Z Y, et al. CircRNA: functions and properties of a novel potential biomarker for cancer[J]. Mol Cancer, 2017, 16(1): 94. DOI:10.1186/s12943-017-0663-2 |




