胰腺坏死感染(infected pancreatic necrosis, IPN)常发生于重型急性胰腺炎(severe acute pancreatitis, SAP)发病3~4周后,多因继发脓毒血症(segticogyemia)、腹腔出血(intra-abdominal hemorrhage)、多器官功能障碍综合征(multiple organ dysfunction syndrome, MODS)、消化道瘘(digestive tract fistula,DTF)等并发症而导致病情加重,造成对胰腺及其他器官第二次打击,是SAP第二次死亡高峰,其病死率为15%~39%[1-3], 更有甚者,有报道病死率高达36%~50%[4-6]。我院消化内科内镜中心自2012年开始,采用内镜下覆膜金属支架置入引流治疗12例IPN和1例PPC患者,获得了较好的临床效果。另外,本研究纳入国内外公开报道的12篇文献中采用覆膜金属支架置入引流治疗患者331例,其中IPN患者193例,胰腺假性囊肿(pancreatic pseudocyst,PPC)患者138例。以我院收治患者为主,结合文献报道病例分析围手术期一般情况、并发症、手术成功率、救治成功率、死亡率、平均住院时间等,旨在评估覆膜金属支架置入术治疗IPN及PPC的疗效,为临床治疗提供参考。
1 资料与方法 1.1 资料来源我院2012-2017年收治IPN患者12例、PPC患者1例,其中男性11例,女性2例,年龄22~64(42.6± 12.1)岁。13例患者术前全部签署知情同意书,均进行内镜下覆膜金属支架置入术。
检索中国知网、万方数据库、维普网、Cochrane Library、PubMed、Web of Science等数据库中2012-2017年公开发表的内镜下覆膜金属支架置入引流治疗IPN及PPC的文献12篇,其中美国7篇,日本2篇,西班牙、澳大利亚和中国各1篇,单个病例报告不纳入本研究;5篇文献研究对象来源于多中心,2篇来源于单中心。12篇文献共纳入内镜下覆膜金属支架置入治疗IPN(193例)及PPC(138例)患者共计331例,其中男性224例,女性107例,年龄10~89岁,平均年龄52.97岁。12篇文献报道的331例与我院收治的13例合计共344例患者,全部进行内镜下覆膜金属支架置入术。
1.2 检查及治疗方法13例患者的处理按照《临床实践指南(2016):急性胰腺炎的处理》[7],术前全部进行心电图检查、胸部X线正侧位片或胸部CT、腹部彩超或腹部CT检查;全部进行血常规、肾功能、肝功能、血糖、血液生化、凝血功能检查及血液或病灶引流物培养。术前全面评估患者的全身情况。采用穿刺超声内镜,术前排除手术禁忌证。采用内镜超声(endoscopic ultrasound, EUS)测定感染灶或囊肿大小、位置和病灶的厚度。在EUS引导下,取病灶向胃明显压迫部位为穿刺区域,胃壁紧邻病灶处为穿刺点,多普勒检查避开血管。从内镜活检孔将穿刺针连同针芯准确刺入病灶腔内。尽量将针头与病灶壁保持垂直,拉近距离快速进针,穿刺时避开血管,拔出针芯,置入黄斑马导丝,采用19 G穿刺针在EUS引导下经胃壁穿刺进入病灶脓腔,在导丝引导下用囊肿切开刀切开胃壁及感染脓腔壁,在X射线监视下置入国产ST74-33 30 mm×16 mm双蘑菇头全覆膜金属支架于胃壁及感染脓腔壁间;经支架置入7.5 F鼻脓肿冲洗管于感染脓腔内,再置入空肠三腔喂养管;以后每天1次或多次用胃镜经支架孔进入感染病灶进行冲洗吸引脓腔,同时清除病灶坏死组织,对于病灶坏死组织范围广泛而不能一次清理完成的重症患者,可多次进行清除引流,直至病灶坏死组织清除完为止,如图 1所示。
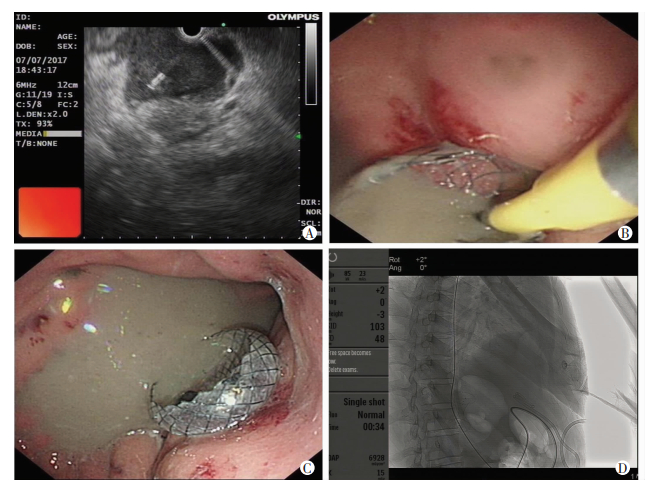
|
| A:超声内镜引导下用19 G穿刺针刺入脓腔内; B:用囊肿切开刀经穿刺路径切开胃-脓肿壁,再置入双蘑菇头全覆膜金属支架,缓慢释放支架,可见大量脓液涌出; C:内镜下显示支架位置; D:X线片显示支架两端扩张良好 图 1 IPN患者覆膜金属支架置入引流术的影像学及内镜下图像s |
1.3 术后观察处理
我院13例患者术后严密观察生命体征变化,如脉搏、呼吸、血压、体温等。术后全部入住GICU,给予一级护理和心电监护。术后给予禁食、抑酸、抑酶、抗感染等对症支持治疗。定期监测血淀粉酶、尿淀粉酶、血常规、凝血功能、动脉血气分析等。床旁复查彩色超声检查,了解感染病灶或假性囊肿消退情况,适时进行腹部CT检查。严密观察是否发生消化道出血、穿孔、术后创伤性胰腺炎复发表现,以及发热、腹痛及肠梗阻等并发症。术后3 d复查胃镜,根据引流情况适当调整支架位置,继续抗感染等对症支持治疗,待引流液减少、病情稳定、症状好转,可以出院,但出院后应嘱患者定期门诊复查、随访。当确定症状完全缓解,感染病灶或胰腺积液及囊肿基本消失后,经内镜取出覆膜金属支架。
1.4 统计学分析采用SPSS 20.0统计软件,计量数据采用x±s表示,计数资料采用例数和构成比表示。
2 结果 2.1 13例IPN、PPC患者覆膜金属支架置入围手术期一般情况13例IPN、PPC患者覆膜金属支架置入围手术期进行了血液和感染病灶穿刺液细菌培养,其中4例血培养为阳性,病原菌为:大肠埃希菌、黏质沙雷菌、白色念珠菌、近平滑假丝酵母菌和光滑假丝酵母菌;另9例感染病灶穿刺液培养阳性,其中肺炎克雷伯菌感染者3例,大肠埃希菌感染者2例,鲍曼不动杆菌感染者2例,热带假丝酵母菌和阴沟肠杆菌感染者各1例。患者覆膜金属支架置入围手术期检测结果见表 1。
| 年龄/岁 | 体温/℃ | 脉搏/次·min-1 | 呼吸/次·min-1 | 收缩血压/mmHg |
| 22~64(42.7 ± 12.1) | 36.3~38.6(37.10 ± 0.7) | 74~154(102.1 ± 23.5) | 14~42(22.8 ± 7.6) | 101~144(122.2 ± 12.7) |
| 白细胞/×109·L-1 | 肌酐/mol·L-1 | 血糖/mmol·L-1 | ALT/IU·L-1 | 白蛋白/g·L-1 |
| 3.2~16.8(8.9 ± 4.5) | 27~74(54.8 ± 13.8) | 5~13(8.1 ± 2.6) | 4~216(37.1 ± 55.7) | 24.4~44.4(33.5 ± 6.1) |
| 总胆红素/mol·L-1 | 直胆红素/mol·L-1 | CT最大直径/cm | CT最小直径/cm | |
| 9.1~122.8(26.9 ± 30.4) | 2.1~61.7(10.1 ± 15.9) | 8.0~26.7(13.0 ± 5.6) | 3.0~11.6(7.8 ± 2.4) | |
2.2 治疗效果
13例患者均成功置入覆膜金属支架。12例IPN患者置入覆膜金属支架后平均经过1.5次(1~3次)内镜下清创,引流管平均每天冲洗3.5次(1~6次),经内镜下覆膜金属支架置入引流及症治疗好转后出院。13例患者平均住院时间为44.8(9~153)d,平均拔出覆膜金属支架时间为18.4(5~45)周,术后并发肺部感染者6例(46.2%),其中1例(7.7%)IPN患者因原发病感染严重,合并脓腔内大出血死亡。12篇文献公开报道的覆膜金属支架置入术后331例患者中,救治成功326例(98.5%),死亡5例(1.5%)。本研究和文献报道共计344例患者,其中救治成功338例(98.3%),死亡6例(1.7%)。
2.3 围术期并发症本研究13例患者中,围术期6例(46.2%)出现肺部感染,1例(7.7%)合并脓腔出血死亡,其余经治疗后好转,未发生支架移位、穿孔、堵管。12篇文献纳入患者中,围术期并发症主要为感染、支架移位及出血,发生率分别为3.9%(13/331)、3.9%(13/331)及3.0%(10/331)。少见的并发症为堵管及穿孔,发生率分别为0.9%(3/331)及0.3%(1/331)。本研究和文献报道共计344例患者中,覆膜金属支架置入治疗IPN及PPC患者术后主要并发症为感染、支架移位、出血(表 2)。
| 作者(年度) | 例数 | 支架移位 | 出血 | 堵管 | 感染 | 穿孔 | 合计 |
| Siddiqui AA(2016)[8] | 82 | 2(2.4%) | 6(7.3%) | 0(0) | 0(0) | 0(0) | 8(9.8%) |
| Walter D(2015)[9] | 61 | 3(4.9%) | 0(0) | 0(0) | 4(6.6%) | 1(1.6%) | 8(13.1%) |
| Chandran S(2015)[10] | 54 | 4(7.4%) | 1(1.9%) | 3(5.6%) | 0(0) | 0(0) | 8(14.8%) |
| Raj JS(2014)[11] | 33 | 1(3.0%) | 0(0) | 0(0) | 2(6.1%) | 0(0) | 3(9.1%) |
| Penn DE(2012)[12] | 20 | 0(0) | 0(0) | 0(0) | 2(10.0%) | 0(0) | 2(10.0%) |
| Itoi T(2012)[13] | 20 | 1(5.0%) | 0(0) | 0(0) | 0(0) | 0(0) | 1(5.0%) |
| Saxena P(2014)[14] | 17 | 0(0) | 0(0) | 0(0) | 1(5.9%) | 0(0) | 1(5.9%) |
| 金震东(2014)[15] | 11 | 1(9.1%) | 0(0) | 0(0) | 2(18.2%) | 0(0) | 3(27.3%) |
| Attam R(2014)[16] | 10 | 0(0) | 1(10.0%) | 0(0) | 2(20.0%) | 0(0) | 3(30.0%) |
| Gornals JB(2012)[17] | 9 | 0(0) | 1(11.1%) | 0(0) | 0(0) | 0(0) | 1(11.1%) |
| Yamamoto N(2013)[18] | 9 | 1(11.1%) | 1(11.1%) | 0(0) | 0(0) | 0(0) | 2(22.2%) |
| Saxena P(2014)[19] | 5 | 0(0) | 0(0) | 0(0) | 0(0) | 0(0) | 0(0) |
| 本研究 | 13 | 0(0) | 1(7.7%) | 0(0) | 6(46.2%) | 0(0) | 7(53.8%) |
| 合计 | 344 | 13(3.8%) | 11(3.2%) | 3(0.9%) | 19(5.5%) | 1(0.3%) | 47(13.7%) |
3 讨论
SAP发生机制十分复杂,临床上15%~20%的急性胰腺炎并发胰腺坏死[20]。早期为急性反应期,中期为坏死感染期,后期为残余感染期。早期临床上主要表现为低血容量、低氧血症和呼吸功能、肝功能及肾功能障碍,以及腹腔间综合征(interabdominal compartment syndrome)等。到了中后期,胰周可大量积液,形成PPC,且由于免疫功能低下,肠黏膜屏障遭到破坏,组织缺血、缺氧,肠黏膜通透性增加,细菌和内毒素向胰腺和胰周坏死组织内易位,导致坏死组织继发感染,形成IPN,进而发生全身炎症反应综合征(sytemic inflammatory response syndrome, SIRS), 使休克进一步加重,严重者发生MODS而导致死亡。IPN一旦明确诊断后,必须积极引流或清创,有望改善SAP患者预后。本研究和文献报道共计344例IPN、PPC患者进行覆膜金属支架置入引流术,338例(98.3%)获得成功救治,死亡仅6例,死亡率为1.7%。表明SAP患者并发IPN、PPC进行覆膜金属支架置入治疗是有效的。
随着医学科学技术的进步和设备的改进,临床上微创内镜下坏死组织引流术或清创术已经广泛应用,并有逐渐替代外科手术清创的趋势[21]。自2009年ANTILLON等[22]报道了首例内镜下经壁引流后置入全覆膜自膨式金属支架治疗包裹性坏死(walled off necrosis, WON)以来,覆膜金属支架置入治疗IPN、PPC在临床应用越来越广。其特点是金属支架直径大,易引流和允许内镜直接通过等优点;其次是与WON和塑料支架比较,全覆膜自膨式金属支架的优势是只需1个支架,在临床应用中大大简化了内镜操作过程。金属支架的直径大,直径通常>10 mm,我们应用的金属支架为30 mm×16 mm,引流速度快,减少了支架阻塞的风险。344例IPN、PPC患者发生阻塞者仅3例,发生率为0.8%。金属支架主要并发症之一是支架移位,但发生率较低,344例患者中发生支架移位者13例,发生率为3.8%;另一个并发症为感染,344例IPN、PPC患者中发生肺部和泌尿系感染等19例[8, 10],发生率为5.5%,特别是我们中心的13例患者中发生肺部感染者达6例之多,发生率高达46.2%,究其原因,可能是患者原发疾病严重。此外,出血也是金属支架置入术的主要并发症,344例IPN、PPC患者中发生出血者为11例,发生率为3.2%。
急性胰腺炎在消化疾病中是第三大常见病,近20%的急性胰腺炎患者发生胰腺或胰周坏死,这与高死亡率有关[4, 23]。在急性胰腺炎发病的第1个月内,坏死组织被纤维外壳包裹形成WON,或称为“急性感染性坏死性胰腺炎”(acute infective necrotizing pancreatitis)。目前对这种坏死感染治疗策略的专家共识是:在早期阶段应避免对胰周液体积聚的干预,直到包裹性液体积聚内含有固体的坏死组织,这通常是在SAP发病的4周之后[24]。与外科手术干预相比,内镜下覆膜金属支架置入引流治疗IPN及PPC因创伤小、疗效好已成为一种新方法。支架用于维持腔内引流,其型号的选择至关重要。大口径金属支架(larger-caliber metal stents)可促进坏死碎片组织自发引流,引流效果好,可使脓(囊)腔较快溶解、缩小。但由于其固定牢靠,不会随脓(囊)腔缩小而向胃腔内移动,增加了与脓(囊)腔壁的摩擦概率,而引起出血[25-26],344例IPN、PPC患者支架置入后发生出血者11例。有研究报道采用覆膜金属支架置入治疗IPN及PPC患者支架通畅率为98.7%(77/78)[8, 27],本文344例覆膜金属支架通畅率为100%。PPC是胰腺周围组织中坏死液体的积聚,四周形成囊壁而不能被吸收。PPC无症状,并会自行溶解或被吸收,然而,当积液面积增大或形成IPN,常会导致患者发热、腹痛、腹部饱胀、胃出口梗阻、胆道梗阻,以及继发黄疸和脓毒血症等。我院内镜中心13例患者病灶最大CT直径为26.7 cm×10.2 cm, 平均直径为(13.0±5.6) cm,最小CT直径也达8.0 cm× 5.0 cm, 平均直径为(7.8±2.4) cm,比文献报道病灶CT直径大[8-9, 15]。
目前对有症状的PPC或IPN患者的治疗方案除抗感染、营养支持治疗外,还包括内镜、外科手术或经皮穿刺引流。外科手术创伤性大,死亡率高;经皮穿刺引流,会有形成瘘管、囊肿复发和增加感染的风险。在过去的10年里,超声内镜引导下支架置入引流术治疗IPN、PPC已成为许多医院的首选方案。采用放置大口径的覆膜金属支架可以有效解决这些患者的临床问题,不良事件少,死亡率低,患者获益颇多[28]。在内镜微创治疗中,选择不同类型内引流支架、减少并发症、提高治疗的安全性及有效性等都值得进一步深入研究。
| [1] |
黄耿文, 申鼎成, 亢浩, 等. 微创腹膜后入路胰腺坏死组织清除术治疗感染性胰腺坏死18例疗效分析[J].
中国实用外科杂志, 2016, 36(11): 1197–1199.
HUANG G W, SHEN D C, KANG H, et al. Minimally invasive retroperitoneal approach for pancreatic necrosis in the treatment of 18 cases of infectious pancreatic necrosis[J]. Chin J Pract Med, 2016, 36(11): 1197–1199. DOI:10.7504/CJPS.ISSN1005-2208.2016.11.16 |
| [2] | VAN GRINSVEN J, VAN BRUNSCHOT S, BAKKER O J, et al. Diagnostic strategy and timing of intervention in infected necrotizing pancreatitis: an international expert survey and case vignette study[J]. HPB(Oxford), 2016, 18(1): 49–56. DOI:10.1016/j.hpb.2015.07.003 |
| [3] |
樊超强, 刘恩, 杨歆, 等. 内镜清创术与外科清创术治疗胰腺坏死感染的回顾性研究[J].
第三军医大学学报, 2017, 39(8): 807–812.
FAN C Q, LIU E, YANG X, et al. Efficacy of endoscopic and surgical necrosectomy for infected pancreatic necrosis: a retrospective study of 68 cases[J]. J Third Mili Med Univ, 2017, 39(8): 807–812. DOI:10.16016/j.1000-5404.201612051 |
| [4] | BANKS P A, BOLLEN T L, DERVENIS C, et al. Classification of acute pancreatitis—2012: revision of the Atlanta classification and definitions by international consensus[J]. Gut, 2013, 62(1): 102–111. DOI:10.1136/gutjnl-2012-302779 |
| [5] | BARON T H, THAGGARD W G, MORGAN D E, et al. Endoscopic therapy for organized pancreatic necrosis[J]. Gastroenterology, 1996, 111(3): 755–764. DOI:10.1053/gast.1996.v111.pm8780582 |
| [6] |
朱勇, 何文华, 夏亮, 等. 内镜下经胃坏死组织清创术治疗重症急性胰腺炎并包裹性坏死的疗效初探[J].
中华消化内镜杂志, 2015, 32(3): 187–190.
ZHU Y, HE W H, XIA L, et al. Endoscopic debridement of gastric necrotic tissue in the treatment of severe acute pancreatitis with encapsulated necrosis[J]. Chin J Digest Endosc, 2015, 32(3): 187–190. DOI:10.3760/cma.j.issn.1007-5232.2015.03.013 |
| [7] | Greenberg J A, Hsu J, Bawazeer M, et al. Clinical practice guideline: management of acute pancreatitis[J]. Can J Surg, 2016, 59(2): 128–140. DOI:10.1503/cjs.015015 |
| [8] | SIDDIQUI A A, ADLER D G, NIETO J, et al. EUS-guided drainage of peripancreatic fluid collections and necrosis by using a novel lumen-apposing stent: a large retrospective, multicenter U.S. experience (with videos)[J]. Gastrointest Endosc, 2016, 83(4): 699–707. DOI:10.1016/j.gie.2015.10.020 |
| [9] | WALTER D, WILL U, SANCHEZ-YAGUE A, et al. A novel lumen-apposing metal stent for endoscopic ultrasound-guided drainage of pancreatic fluid collections: a prospective cohort study[J]. Endoscopy, 2015, 47(1): 63–67. DOI:10.1055/s-0034-1378113 |
| [10] | CHANDRAN S, EFTHYMIOU M, KAFFES A, et al. Management of pancreatic collections with a novel endoscopically placed fully covered self-expandable metal stent: a national experience (with videos)[J]. Gastrointest Endosc, 2015, 81(1): 127–135. DOI:10.1016/j.gie.2014.06.025 |
| [11] | SHAH R J, SHAH J N, WAXMAN I, et al. Safety and efficacy of endoscopic ultrasound-guided drainage of pancreatic fluid collections with lumen-apposing covered self-expanding metal stents[J]. Clin Gastroenterol Hepatol, 2015, 13(4): 747–752. DOI:10.1016/j.cgh.2014.09.047 |
| [12] | PENN D E, DRAGANOV P V, WAGH M S, et al. Prospective evaluation of the use of fully covered self-expanding metal stents for EUS-guided transmural drainage of pancreatic pseudocysts[J]. Gastrointest Endosc, 2012, 76(3): 679–684. DOI:10.1016/j.gie.2012.04.457 |
| [13] | ITOI T, BINMOELLER K F, SHAH J, et al. Clinical evaluation of a novel lumen-apposing metal stent for endosonography-guided pancreatic pseudocyst and gallbladder drainage (with videos)[J]. Gastrointest Endosc, 2012, 75(4): 870–876. DOI:10.1016/j.gie.2011.10.020 |
| [14] | SARKARIA S, SETHI A, RONDON C, et al. Pancreatic necrosectomy using covered esophageal stents: a novel approach[J]. J Clin Gastroenterol, 2014, 48(2): 145–152. DOI:10.1097/MCG.0b013e3182972219 |
| [15] |
金震东, 蒋斐, 姚瑶, 等. 内镜超声引导下经胃穿刺覆膜金属支架引流胰腺假性囊肿的初步临床研究[J].
中华消化内镜杂志, 2014, 31(9): 486–488.
JIN Z D, JIANG F, YAO Y, et al. Clinical evaluation of fully covered self-expanding metal stent for endosonograph-guided transgastric pancreatic pseudocyst drainage[J]. Chin J Digest Endosc, 2014, 31(9): 486–488. DOI:10.3760/cma.j.issn.1007-5232.2014.09.002 |
| [16] | ATTAM R, TRIKUDANATHAN G, ARAIN M, et al. Endoscopic transluminal drainage and necrosectomy by using a novel, through-the-scope, fully covered, large-bore esophageal metal stent: preliminary experience in 10 patients[J]. Gastrointest Endosc, 2014, 80(2): 312–318. DOI:10.1016/j.gie.2014.02.013 |
| [17] | GORNALS J B, DE LA SERNA-HIGUERA C, SÁNCHEZ-YAGUE A, et al. Endosonography-guided drainage of pancreatic fluid collections with a novel lumen-apposing stent[J]. Surg Endosc, 2013, 27(4): 1428–1434. DOI:10.1007/s00464-012-2591-y |
| [18] | YAMAMOTO N, ISAYAMA H, KAWAKAMI H, et al. Preliminary report on a new, fully covered, metal stent designed for the treatment of pancreatic fluid collections[J]. Gastrointest Endosc, 2013, 77(5): 809–814. DOI:10.1016/j.gie.2013.01.009 |
| [19] | SAXENA P, SINGH V K, MESSALLAM A, et al. Resolution of walled-off pancreatic necrosis by EUS-guided drainage when using a fully covered through-the-scope self-expandable metal stent in a single procedure (with video)[J]. Gastrointest Endosc, 2014, 80(2): 319–324. DOI:10.1016/j.gie.2014.04.041 |
| [20] | DA COSTA D W, BOERMA D, VAN SANTVOORT H C, et al. Staged multidisciplinary step-up management for necrotizing pancreatitis[J]. Br J Surg, 2014, 101(1): e65–e79. DOI:10.1002/bjs.9346 |
| [21] | IAP/APA. evidence-based guidelines for the management of acute pancreatitis[J]. Pancreatology, 2013, 13(4 Suppl 2): e1–15. DOI:10.1016/j.pan.2013.07.063 |
| [22] | ANTILLON M R, BECHTOLD M L, BARTALOS C R, et al. Transgastric endoscopic necrosectomy with temporary metallic esophageal stent placement for the treatment of infected pancreatic necrosis (with video)[J]. Gastrointest Endosc, 2009, 69(1): 178–180. DOI:10.1016/j.gie.2008.03.1066 |
| [23] | PEERY A F, CROCKETT S D, BARRITT A S, et al. Burden of gastrointestinal, liver, and pancreatic diseases in the United States[J]. Gastroenterology, 2015, 149(7): 1731–1741. DOI:10.1053/j.gastro.2015.08.045 |
| [24] | FREEMAN M L, WERNER J, VAN SANTVOORT H C, et al. Interventions for necrotizing pancreatitis: summary of a multidisciplinary consensus conference[J]. Pancreas, 2012, 41(8): 1176–1194. DOI:10.1097/MPA.0b013e318269c660 |
| [25] | VAN SANTVOORT H C, BESSELINK M G, BAKKER O J, et al. A step-up approach or open necrosectomy for necrotizing pancreatitis[J]. N Engl J Med, 2010, 362(16): 1491–1502. DOI:10.1056/NEJMoa0908821 |
| [26] | BANG J Y, HASAN M, NAVANEETHAN U, et al. Lumen-apposing metal stents (LAMS) for pancreatic fluid collection (PFC) drainage: may not be business as usual[J]. Gut, 2017, 66(12): 2054–2056. DOI:10.1136/gutjnl-2016-312812 |
| [27] | VARADARAJULU S, RANA S S, BHASIN D K. Endoscopic therapy for pancreatic duct leaks and disruptions[J]. Gastrointest Endosc Clin N Am, 2013, 23(4): 863–892. DOI:10.1016/j.giec.2013.06.008 |
| [28] | BAZERBACHI F, SAWAS T, VARGAS E J, et al. Metal stents versus plastic stents for the management of pancreatic walled-off necrosis: a systematic review and meta-analysis[J]. Gastrointest Endosc, 2018, 87(1): 30–42. DOI:10.1016/j.gie.2017.08.025 |



