2. 400010 重庆, 重庆医科大学:超声分子影像重庆市重点实验室
2. Chongqing Key Laboratory of Ultrasound Molecular Imaging, Chongqing Medical University, Chongqing, 400010, China
目前对急性肺损伤/急性呼吸窘迫综合征(acute lung injury/acute respiratory distress syndrome, ALI/ARDS)的临床诊疗水平有所提高,但无法达到满意的疗效,病死率依然较高[1-2]。
血管内皮细胞功能障碍在ARDS的发生、发展中居重要作用。最新研究发现血管内皮细胞功能障碍和非受体酪氨酸家族激酶受体位点Arg和c-Abl相关[3],这些激酶可能参与调节内皮细胞的粘附、增殖、分化和迁移[4]。伊马替尼是一种非受体酪氨酸蛋白激酶Arg和c-Abl抑制剂,用于治疗费城染色体阳性慢性髓性白血病及其他恶性肿瘤[5]。已经有研究提示伊马替尼在小鼠模型中有抗炎和抗纤维化作用[6],也有研究提示伊马替尼对肺有保护作用[7]。但是伊马替尼副作用较大,常规剂量会引起恶心、肌痛和骨水肿等副作用[8-9]。
脂质体能够将药物选择性的聚集于靶器官或组织,有效减少药物副反应。ICAM-1是相对分子量为(74~114)×103的Ⅰ型跨膜蛋白,主要表达于Ⅱ型肺泡上皮细胞。液态氟碳在体内温度条件下可发生液气相变。我们选择使用ICAM-1抗体进一步提高药物的靶向聚集,观察靶向抗ICAM-1的载伊马替尼和液态氟碳脂质体对LPS诱导的ALI/ARDS小鼠模型的治疗效果,以及在各个靶器官的分布。
1 材料与方法 1.1 实验动物30只6~8周大小C57BL/6小鼠,雌雄随机,体质量20~25 g,购自重庆医科大学实验动物中心,并在动物中心代养。
1.2 实验试剂甲磺酸伊马替尼(IM),内毒素(LPS)购自美国Sigma公司。IL-6,IL-8,IL-10,TNF-α引物由上海生工合成。1-棕榈酰基-2-硬脂酰基卵磷脂(HSPC),二硬脂酰磷脂酰乙醇胺-聚乙二醇2000-生物素(DSPE-PEG2000-Biotin),二棕榈酰磷脂酰甘油(DPPG),胆固醇(Cholesterol)购自瑞士Corden公司, ati-ICAM-1-Biotin购自北京博奥森生物有限公司。TRIzol试剂盒购自美国Invitrogen公司,荧光定量PCR试剂盒购自日本TaKaRa公司。链霉素,细胞膜红色荧光探针(Dil),异硫氰酸荧光素(FITC)等其他试剂均购于上海碧云天生物技术有限公司。
1.3 靶向抗ICAM载伊马替尼和液态氟碳脂质体制备HSPC,DPPG,DSPE-PEG2000-Biotin, Cholesterol以一定的比例溶在10 mL氯仿中,装入圆底烧瓶,瓶口密封;完全溶解后,将圆底烧瓶置于旋转蒸发仪50 ℃减压蒸发2 h蒸干氯仿,转速80 r/min;待圆底烧瓶底部形成均匀脂膜后加入PBS溶液,将其置于恒温箱内缓慢振荡直至水化完全;冰浴条件下,声振仪乳化时间为8 min,乳化过程中同时逐滴加入液态氟碳和伊马替尼,最后得到乳白色混悬液,即为载药液态氟碳纳米粒;在4 ℃条件下将纳米粒离心洗涤2次,保存于4 ℃。加入链霉素孵育一段时间,并与anti-ICAM-1-Biotin孵育。
1.4 载伊马替尼和液态氟碳脂质体体外相变和体外药物释放曲线光镜下观察脂质体,置于37.5 ℃的恒温加热板上。在透析袋内加入4 mL载伊马替尼和液态氟碳脂质体,放入50 mL烧杯中,加入30 mL PBS缓冲液(pH=7.4),将烧杯放在37.5 ℃恒温水浴箱振荡(转速100 r/min);设置取样时间点1、2、4、6、12、24 h,每次取样2 mL,取完后补回2 mL PBS缓冲液。测量取出的药物浓度,计算药物释放率。
1.5 实验动物分组及ALI/ARDS模型构建采用随机数字表法将30只C57BL/6小鼠分为3组,每组10只:Ⅰ组为对照组;Ⅱ组为LPS造模组;Ⅲ组为靶向抗ICAM-1载伊马替尼(IM)脂质体治疗组。其中Ⅰ组尾静脉注射100 μL PBS;Ⅱ组气管给予50 μL LPS(1 mg/kg),尾静脉注射100 μL PBS;Ⅲ组气管给予50 μL LPS(1 mg/kg),尾静脉注射100 μL靶向抗ICAM-1脂质体。取肺组织4%多聚甲醛固定,用肺组织做石蜡切片HE染色。同时取各组老鼠器官新鲜样本,做冰冻切片,共聚焦观察脂质体在各个器官冰冻切片的分布。
1.6 ALI/ARDS的病理切片染色评分系统及标准[10]肺组织石蜡切片HE染色观察。评分标准:(1)肺泡出血;(2)肺泡水肿;(3)肺泡腔中性粒细胞浸润或聚集;(4)肺泡壁增厚和透明膜形成及炎症细胞浸润。依据病变轻重评分(0分为无病变;1分为轻度病变;2分为中度病变;3分为重度病变;4分为极重度病变)。ARDS总评分为各项评定总和。
1.7 小鼠肺湿干重比(W/D)称量肺湿重,烤箱烤干到恒重称量得到干重。根据公式计算肺W/D值。
1.8 qPCR法测定小鼠肺组织依据RNA提取试剂盒说明书提取小鼠肺组织总mRNA,测量RNA浓度,反转录为cDNA。随后按照扩增试剂盒说明书进行PCR扩增,TNF-α上游引物为5′-CGAGTGACAAGCCCGTAGCC-3′,下游引物为5′-GG-ATGAACACGCCAGTCGCC-3′;IL-6上游引物为5′-GCA-CTGGCAGAAAACAACCT-3′,下游引物5′-TCAAACTCCAAAAGACCAGTGA-3′。IL-8上游引物为5′-CCAACACAGAAATTATTGTAAAGC-3′,下游引物5′-TGAATTCTCAGCCCTCTTCAA-3′,IL-10上游引物为5′-AGCTGGACAACATACTGC-3′,下游引物5′-TCATTCATGGCCTTGTAG-3′,内参β-actin上游引物5’-CGAGCGGGCTACAGCTTC-3’,下游引物为5′-GTCACGCACGATTCCCTCT-3’。PCR反应条件:94 ℃ 5 min;94 ℃ 30 s,57 ℃ 30 s,72 ℃ 60 s,72 ℃ 10 min,共35个循环。
1.9 小鼠肺泡液体清除率(alveolar fluid clearance,AFC)气管滴注4%伊文氏蓝标记白蛋白生理盐水,机械通气后,测定肺泡白蛋白浓度,并计算AFC。AFC(%)=(Vi-Vf)/Vi×100%,Vf=Vi×Pi/Pf,其中Vi为注入肺泡内液体的量,Vf为肺泡内剩余的液体量,Pi为最初白蛋白浓度,Pf为最终的白蛋白浓度。
1.10 统计学方法采用SPSS 13统计软件分析,计量资料以x±s表示。其两两比较采用LSD-t法,检验水准α=0.05。
2 结果 2.1 载伊马替尼脂质体的基本特性及在模拟体内环境的体外释放曲线光镜下观察靶向抗ICAM-1的载伊马替尼和液态氟碳脂质体,分布均匀,大小粒径为(320.8±58.7)nm,电镜下观察到脂质体内部有黑色颗粒药物。在37.5 ℃的恒温板上,观察到液态氟碳的相变,显微镜下见脂质体逐渐变大形成微泡,最后破裂。Dil染的脂质体发出红色荧光。用FITC标记连靶脂质体靶点抗体,观察到脂质体发出蓝色荧光。同时,体外模拟人体环境中,随着时间的延长,药物缓慢释放,2 h药物释放率约40.6%,12 h后药物释放率约75.8%,开始进入平台期,24 h后药物几乎停止释放(图 1)。
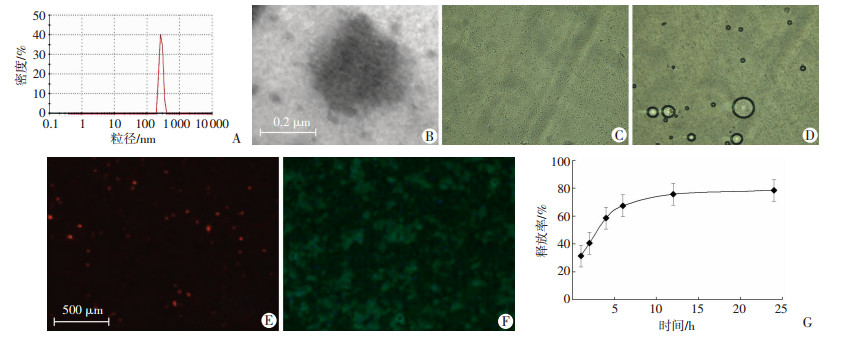
|
| A:靶向抗ICAM-1的载伊马替尼和液态氟碳脂质体的粒径分布;B:透射电镜观察;C:脂质体的光镜观察(×200);D:在37.5 ℃加热板上脂质体相变光镜观察(×200);E:脂质体红色荧光观察;F:靶向抗ICAM-1脂质体连接FITC发出绿色荧光(×200);G:在模拟体内环境下,24 h靶向脂质体药物的释放曲线 图 1 载伊马替尼脂质体的基本形态及在模拟体内环境的体外释放特征 |
2.2 ICAM-1在各组小鼠肺中的表达
免疫荧光观察ICAM-1在肺的表达情况(图 2),与对照组相比,LPS造模组ICAM-1表达明显增加,且ICAM-1在肺的表达随炎症的加重而增多。在靶向抗ICAM-1脂质体治疗组,ICAM-1表达比对照组增加。
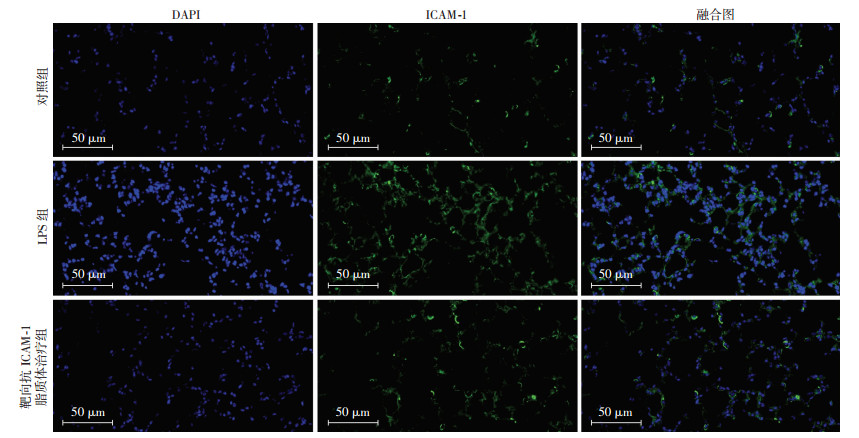
|
| 绿色荧光示ICAM-1表达,DAPI染细胞核 图 2 免疫荧光染色观察ICAM-1在3组小鼠肺组织中的表达 |
2.3 靶向抗ICAM-1脂质体对LPS诱导的小鼠模型治疗效果评价
对照组小鼠精神状态佳,呼吸平稳,双肺正常,肺组织切片无明显病理变化;LPS造模组小鼠出现精神萎靡,呼吸急促,双肺肿胀,明显出血斑,肺病理HE染色切片可见肺泡间隔明显增厚,肺泡充血水肿,大量炎症细胞浸润;靶向抗ICAM-1脂质体治疗组小鼠精神状态和离体肺肉眼观察均明显好转,切片可见肺泡间隔轻度增厚伴少量炎性细胞浸润,肺泡轻度出血水肿。通过肺的HE染色切片评分比较,我们发现LPS造模组平均切片评分(14分)与对照组(2分)相比增高(P < 0.01),靶向抗ICAM-1脂质体治疗后,炎症有所减缓,平均评分(7分)下降(P < 0.05)(图 3)。与对照组相比,LPS造模组IL-6,IL-8,TNF-α mRNA,小鼠W/D明显升高(P < 0.01),IL-10 mRNA和AFC明显降低(P < 0.01)。靶向抗ICAM-1脂质体对LPS诱导的小鼠肺IL-6,IL-8,TNF-α mRNA和小鼠W/D升高有明显抑制作用(P < 0.01),IL-10和AFC结果则上升(P < 0.05)。见图 4。

|
| 图 3 3组小鼠肺石蜡切片HE染色观察 |

|
| A:IL-6 mRNA;B: IL-8 mRNA;C: TNF-α mRNA;D: IL-10 mRNA;E:肺湿干重比;F:肺泡液体清除率;a:P < 0.01,与对照组比较;b :P < 0.05, 与LPS造模组比较 图 4 3组小鼠各炎症因子mRNA的表达及肺湿干重比和肺泡液体清除率的比较 |
2.4 评价靶向抗ICAM-1脂质体和无靶脂质体在ALI/ARDS小鼠各个器官的分布
对LPS诱导的ALI/ARDS小鼠尾静脉注射靶向抗ICAM-1脂质体和非靶向脂质体,24 h后取肝、心、脾、肺、肾做冰冻切片,共聚焦激光显微镜观察显示脂质体集中在肺、脾、肝,与非靶向脂质体治疗组相比,靶向抗ICAM-1脂质体治疗组中,脂质体在肺部的分布比例更多(图 5),说明靶向抗ICAM-1脂质体能够将药物集中在靶器官肺。
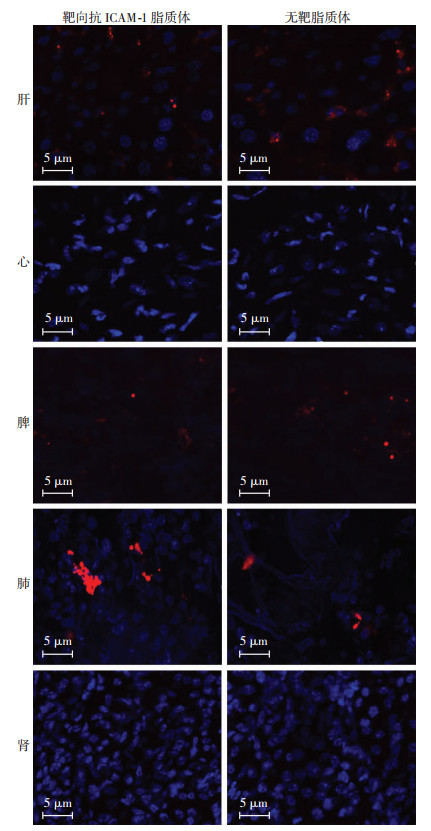
|
| 蓝色:DAPI染核,红色: Dil染脂质体 图 5 共聚焦下观察脂质体在各个器官分布 |
3 讨论
ALI/ARDS的致病环节中,血管内皮细胞不仅是炎症反应损害的主要靶细胞,同时也在炎症级联反应中发挥炎性细胞的作用。内毒素等外界致病因子作用下,肺血管内皮出现肿胀、通透性改变以及胞内颗粒分泌增多等形态和功能的改变,继而发生凋亡,损伤脱落,破坏血管完整性。由于血管内皮细胞的损伤,使内皮下膜暴露,白细胞和单核细胞的聚集黏附于血管内皮表面,形成小栓子,加重肺组织的缺血缺氧[11-13]。
有研究提示血管内皮受体位点Arg和c-Abl抑制剂可以抑制多种炎症因子的转录,减少炎性因子释放,降低血管通透性,减轻血管渗漏,恢复血管内皮屏障功能[14-16]。本实验结果提示Arg和c-Abl抑制剂伊马替尼对LPS诱导的ALI/ARDS小鼠有显著的保护作用,抑制促炎因子作用,增加抑炎因子表达。伊马替尼可以降低肺血管通透性,减缓炎症性血管渗漏,有效减轻ALI/ARDS的损伤。故伊马替尼有望通过保护血管内皮细胞为ALI/ARDS的治疗提供新的思路。
但是伊马替尼作为抗肿瘤药,副作用大,而ALI/ARDS患者多伴有不同程度的多个器官功能损害,难以耐受副作用较大的药物。因此,我们使用纳米脂质体来减轻伊马替尼的副作用,对ARDS进行靶向药物治疗。纳米脂质体首次进入重症监护医疗领域是1990年美国食品药品监督管理局批准的两性霉素脂质体制剂[17]。此后,纳米药物阿米卡星脂质体用于治疗支气管扩张症,表现出临床应用价值[18]。纳米载体应用的关键是提高载体的靶向性,MUZYKANTOV等[19-20]发现通过连接抗体可以显著提高纳米脂质体的靶向药物聚集。
本实验以ICAM-1作为脂质体的靶点,成功将靶向抗ICAM-1脂质体靶向运输至靶器官肺。同时,我们在实验中通过液态氟碳的相变达到在人体环境中释放药物的目的。液态氟碳常温常压下沸点为29.2 ℃,在体内温度条件下,载液态氟碳脂质体可以发生液气相变形成脂质微泡,最后破裂释放药物。我们研究提示靶向脂质体的靶点ICAM-1在肺炎症部位表达增强,在正常的肺区表达较低。在小鼠动物ALI/ARDS模型中,通过尾静脉注射靶向抗ICAM-1脂质体,靶向抗ICAM-1脂质体表面的ICAM-1抗体可与肺炎区明显高表达的ICAM-1抗原高亲和力结合,发挥主动靶向性,显著提高伊马替尼对ALI/ARDS的靶向治疗作用。在此基础上,脂质体同时可以通过缺氧性血管收缩分流到达远端的肺泡,最终在肺的分布与ALI/ARDS小鼠空间异质性的病理相符合。
| [1] | BERNARD G R, ARTIGAS A, BRIGHAM K L, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination[J]. Am J Respir Crit Care Med, 1994, 149(3 Pt 1): 818–824. DOI:10.1164/ajrccm.149.3.7509706 |
| [2] | WARE L B, MATTHAY M A. The acute respiratory distress syndrome[J]. N Engl J Med, 2000, 342(18): 1334–1349. DOI:10.1056/NEJM200005043421806 |
| [3] | CHISLOCK E M, RING C, PENDERGAST A M. Abl kinases are required for vascular function, Tie2 expression, and angiopoietin-1-mediated survival[J]. Proc Natl Acad Sci USA, 2013, 110(30): 12432–12437. DOI:10.1073/pnas.1304188110 |
| [4] | GREUBER E K, SMITH-PEARSON P, WANG J, et al. Role of ABL family kinases in cancer:from leukaemia to solid tumours[J]. Nat Rev Cancer, 2013, 13(8): 559–571. DOI:10.1038/nrc3563 |
| [5] | MOEN M D, MCKEAGE K, PLOSKER G L, et al. Imatinib:a review of its use in chronic myeloid leukaemia[J]. Drugs, 2007, 67(2): 299–320. DOI:10.2165/00003495-200767020-00010 |
| [6] | RHEE C K, LEE S H, YOON H K, et al. Effect of nilotinib on bleomycin-induced acute lung injury and pulmonary fibrosis in mice[J]. Respiration, 2011, 82(3): 273–287. DOI:10.1159/000327719 |
| [7] | AMAN J, PETERS M J, WEENINK C, et al. Reversal of vascular leak with imatinib[J]. Am J Respir Crit Care Med, 2013, 188(9): 1171–1173. DOI:10.1164/rccm.201301-0136LE |
| [8] | DOS SANTOS L V, LIMA J P, ABDALLA K C, et al. Imatinib-induced bone edema:case report and review of literature[J]. J Natl Compr Canc Netw, 2013, 11(10): 1187–1191. DOI:10.6004/jnccn.2013.0140 |
| [9] | DRUKER B J, TALPAZ M, RESTA D J, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia[J]. N Engl J Med, 2001, 344(14): 1031–1037. DOI:10.1056/NEJM200104053441401 |
| [10] | WANG Q, ZHENG X, CHENG Y, et al. Resolvin D1 stimulates alveolar fluid clearance through alveolar epithelial sodium channel, Na, K-ATPase via ALX/cAMP/PI3K pathway in lipopolysaccharide-induced acute lung injury[J]. J Immunol, 2014, 192(8): 3765–3777. DOI:10.4049/jimmunol.1302421 |
| [11] |
刘俊彦, 吕学军, 赵维, 等. Sirt1通过去乙酰化NF-κB/p65减轻小鼠肺泡Ⅱ型上皮细胞脂多糖损伤[J].
第三军医大学学报, 2017, 39(14): 1415–1421.
LIUJ Y, LYU X J, ZHAO W, et al. Sirt1 alleviated lipopolysaccharide damage in alveolar type Ⅱ epithelial cells by deacetylated NF-kappa B/p65 in[J]. J Third Mil Med Univ, 2017, 39(14): 1415–1421. DOI:10.16016/j.1000-5404.201611089 |
| [12] |
梁家宁, 周倩倩, 张天相, 等. TNF-α和IL-1β在急性呼吸窘迫综合征小鼠肺组织表达的变化及评价[J].
细胞与分子免疫学杂志, 2017, 33(2): 159–163.
LIANGJ N, ZHOU Q Q, ZHANG T X, et al. Changes of TNF-alpha and IL-1 beta in lung tissues of mice with acute respiratory distress syndrome and evaluation of[J]. Cell Mol Immunol, 2017, 33(2): 159–163. DOI:10.13423/j.cnki.cjcmi.008006 |
| [13] |
王川江, 徐昉. 过度自噬对白介素-1β在急性呼吸窘迫综合征中失控性炎症的调节作用研究[J].
重庆医科大学学报, 2017, 42(1): 15–20.
WANGC J, XU F. Excessive autophagy on interleukin-1 beta in acute respiratory distresssyndrome in the comprehensive regulation of inflammatory effect[J]. J Chongqing Med Univ, 2017, 42(1): 15–20. DOI:10.13406/j.cnki.cyxb.001144 |
| [14] | LETSIOU E, RIZZO A N, SAMMANI S, et al. Differential and opposing effects of imatinib on LPS-and ventilator-induced lung injury[J]. Am J Physiol Lung Cell Mol Physiol, 2015, 308(3): L259–L269. DOI:10.1152/ajplung.00323.2014 |
| [15] | CHANDEL N S, BUDINGER G R, MUTLU G M, et al. Keratinocyte growth factor expression is suppressed in early acute lung injury/acute respiratory distress syndrome by smad and c-Abl pathways[J]. Crit Care Med, 2009, 37(5): 1678–1684. DOI:10.1097/CCM.0b013e31819fc81a |
| [16] | MITSOPOULOS P, OMRI A, ALIPOUR M, et al. Effectiveness of liposomal-N-acetylcysteine against LPS-induced lung injuries in rodents[J]. Int J Pharm, 2008, 363(1/2): 106–111. DOI:10.1016/j.ijpharm.2008.07.015 |
| [17] | STEIMBACH L M, TONIN F S, VIRTUOSO S, et al. Efficacy and safety of amphotericin B lipid-based formulations-A systematic review and meta-analysis[J]. Mycoses, 2017, 60(3): 146–154. DOI:10.1111/myc.12585 |
| [18] | OLIVIER K N, GRIFFITH D E, EAGLE G, et al. Randomized trial of liposomal amikacin for inhalation in nontuberculous Mycobacterial lung disease[J]. Am J Respir Crit Care Med, 2017, 195(6): 814–823. DOI:10.1164/rccm.201604-0700OC |
| [19] | MUZYKANTOV V R, CHRISTOFIDOU-SOLOMIDOU M, BALYASNIKOVA I, et al. Streptavidin facilitates internalization and pulmonary targeting of an anti-endothelial cell antibody (platelet-endothelial cell adhesion molecule 1):a strategy for vascular immunotargeting of drugs[J]. Proc Natl Acad Sci USA, 1999, 96(5): 2379–2384. DOI:10.1073/pnas.96.5.2379 |
| [20] | BRENNER J S, BHAMIDIPATI K, GLASSMAN P M, et al. Mechanisms that determine nanocarrier targeting to healthy versus inflamed lung regions[J]. Nanomedicine, 2017, 13(4): 1495–1506. DOI:10.1016/j.nano.2016.12.019 |



