2.550002 贵阳,贵州省人民医院:神经内科
2. Department of Neurology, Guizhou Provincial People's Hospital, Guiyang, Guizhou Province, 550002, China
“糖尿病脑病”由Nielon于1965年提出,它以获得性认知和行为缺陷为特征,涵盖了病理、影像、电生理、神经生化、神经心理和行为学等多方面的改变。目前糖尿病脑病的发病机制未明,假说主要集中在高血糖引起的直接损伤、氧化应激、大脑胰岛素信号通路异常等。另外有尸检和流行病学研究报道糖尿病脑病患者血液和大脑中炎症因子都有明显的升高,提示神经系统内炎症反应的过度激活可能也是糖尿病脑病病理机制的重要参与因素[1-3]。线粒体是细胞内的供能中心,也是氧自由基产生的关键部位,线粒体在中枢神经系统损伤后神经细胞存活或死亡过程中起关键作用,因为它调控着能量代谢和凋亡通路[4-7],线粒体的损伤及功能障碍与糖尿病脑病发生发展密切相关[8]。如何保护糖尿病脑病脑线粒体的功能是当前研究的热点问题,本研究旨在探讨颈交感神经阻滞对糖尿病大鼠脑线粒体功能的影响及其可能机制。
1 材料与方法 1.1 实验动物健康成年清洁级雄性SD大鼠40只,8周龄,体质量(250±20) g(第三军医大学动物实验室提供,许可证号SCXK2012-0005)。大鼠笼盒饲养,自由进食、饮水。
1.2 动物分组及建模动物分组:数字随机法分为3组:① 对照组(n=10);② 糖尿病组(n=15);③ 糖尿病+颈交感神经阻滞组(n=15)。大鼠禁食12 h,糖尿病组和阻滞组按65 mg/kg剂量腹腔1次注射1%STZ(溶于pH=4.2,0.1 mol/L柠檬酸一柠檬酸钠缓冲液),血糖仪监测尾静脉血血糖,血糖稳定3 d后,血糖>16.67 mmol/L糖尿病模型制作成功[5],对照组腹腔注射等量缓冲液,成模后8周阻滞组给予颈交感神经阻滞治疗,注射0.25%的布比卡因0.15 mL每天2次(左、右各1次),连续干预2周,于第12周末处死大鼠。
1.3 Morris水迷宫测试 1.3.1 实验方法Morris水迷宫为直径120 cm、高60 cm的圆形水池。平台(直径9 cm,高30 cm,圆形)随机放置于任意一象限,限离池壁30 cm,水温22~25 ℃。从第12周开始训练,每天1次,前4天为训练,第5天为测试。
1.3.2 定位航行试验用于测定大鼠空间学习和记忆能力选择一个象限作为入水点,观察并记录大鼠爬上平台的轨迹图、游泳速度及所需时间(潜伏期)。如果大鼠在60 s内未找到平台,需将其引导至平台并在平台上站20 s,此时潜伏期记为60 s。
1.3.3 空间探索试验用于测定大鼠对原平台的记忆能力训练大鼠几次后撤平台,将大鼠从某一象限池壁中点面向池壁放入水中,记录60 s内大鼠穿越原平台位置的次数。
1.4 线粒体呼吸功能测定取大鼠新鲜海马脑组织,称量后放入预冷的Dounce匀浆器。以1 :10体积加入线粒体分离介质(230 mmol/L甘露醇,1 mmol/L EDTA,70 mmol/L蔗糖,0.25%BSA,Tris-Hcl调pH值至7.4)。匀浆10次至无脑组织块。800 r/min低温离心4 min,取上清液:1 600 r/min低温离心15 min,弃上清,沉淀即为提取的线粒体。按1 :0.4体积加入分离介质重悬线粒体,置于冰槽保存待检。整个操作在0~4 ℃冰浴中进行。采用Clark氧电极法测定线粒体呼吸功能[5]。反应槽内加入2.5 mL反应介质(225 mmol/L甘露醇,1 mmol/L EDTA,70 mmol/L蔗糖,0.1%BSA,10 mmol/L磷酸钾,温度25 ℃,pH=7.4),充分搅拌使基础氧浓度达到240 nmol/mL稳定状态。加入20 μL(约2 mg)线粒体悬液孵育。加入20 μL底物(4 mmol/L琥珀酸二钠),测得的氧消耗率为Ⅳ态呼吸(R4)。再加入20 μL二磷酸腺苷(ADP,50 mmol/L),测得氧消耗率为Ⅲ态呼吸(R3)。线粒体呼吸控制率RCR=R3/R4。
1.5 免疫组化染色取海马组织石蜡切片、脱蜡、自来水洗1 min,抗原修复热修复20 min(中杉ZL1-9065柠檬酸盐缓冲液)、PBS洗3次各3 min,3%H2O2滴加在切片上,室温静置10 min,PBS洗3次各5 min。滴加兔血清工作液室温封闭15 min,甩去多余液体,滴加一抗,4 ℃过夜TNF-α (兔来源)稀释度1 :250(5 μg/mL),IL-6(小鼠来源)稀释度1 :400(1 μg/mL),室温复温,PBS洗3次各5 min,滴加二抗工作液;37 ℃孵育30 min,PBS洗3次各5 min,滴加辣根酶标记链霉卵白素工作液,37 ℃孵育30 min,PBS洗3次各5 min。DAB(中杉金桥ZL1-9018) 显色,在显微镜下掌握染色程度。PBS浸泡10 min;苏木精复染1 min,自来水冲洗,盐酸酒精分化5 s;自来水冲洗10 min;脱水、透明、封片、镜检。
1.6 统计学分析均采用SPSS17.0统计软件进行分析,计量资料以x±s表示,采用单因素方差分析。
2 结果 2.1 3组大鼠认知功能的比较糖尿病组大鼠在12周时存活12只,阻滞组存活13只,行为学测试与对照组比较,糖尿病组、阻滞组逃避潜伏期明显延长、穿越平台的次数明显减少(P<0.05)。与糖尿病组比较,阻滞组大鼠逃避潜伏期缩短、穿越平台的次数增加,但差异无统计学意义(P>0.05,表 1)。
| 组别 | n | 逃避潜伏期(s) | 穿越平台次数(/min) |
| 对照组 | 10 | 25.62±2.84 | 6.32±1.51 |
| 糖尿病组 | 12 | 40.22±5.27a | 3.12±1.16a |
| 阻滞组 | 13 | 38.88±5.31a | 3.52±1.21a |
| a:P<0.05,与对照组比较 | |||
2.2 线粒体呼吸功能的比较
Clark氧电极测定呼吸功能结果显示,糖尿病组和阻滞组的线粒体呼吸功能均较对照组明显下降,主要表现为R3和RCR值显著降低(P<0.05);但阻滞组的R3和RCR高于B组(P<0.05,表 2)。
| 组别 | n | R3 | R4 | RCR |
| 对照组 | 10 | 66.31±5.65 | 12.97±3.38 | 4.78±0.68 |
| 糖尿病组 | 12 | 28.51±3.01a | 13.93±1.16a | 2.09±0.27a |
| 阻滞组 | 13 | 45.26±4.21ab | 11.99±1.11ab | 3.52±0.32ab |
| a:P<0.05,与对照组比较;b:P<0.05,与糖尿病组比较 | ||||
2.3 3组大鼠海马CA1区免疫组化染色结果
阳性结果判定:海马CA1区的TNF-α及IL-6阳性细胞表达为细胞质呈棕黄色,细胞核呈浅蓝色或紫蓝色。IL-6、TNF-α染色结果:在海马CA1区,糖尿病组和阻滞组的IL-6、TNF-α表达均高于对照组(P<0.05)。但阻滞组又明显低于糖尿病组,差异有统计学意义(P<0.05,表 3、图 1)。
| 组别 | n | IL-6 | TNF-α |
| 对照组 | 10 | 13.87±2.54 | 11.09±8.87 |
| 糖尿病组 | 12 | 53.32±6.29a | 63.21±13.24a |
| 阻滞组 | 15 | 28.14±3.82ab | 32.76±5.98ab |
| a:P<0.05,与对照组比较;b:P<0.05,与糖尿病组比较 | |||
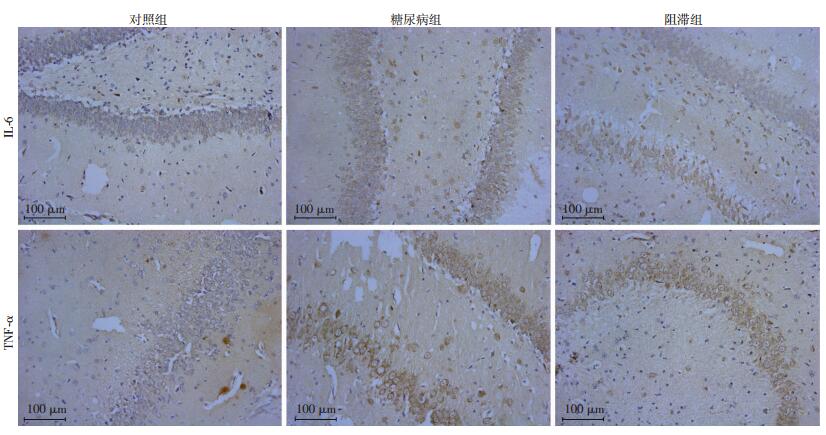
|
| 图 1 3组大鼠海马CA1区TNF-α及IL-6免疫组化染色结果(二步法) |
3 讨论
颈交感神经阻滞(stellate ganglion block,SGB)是将局部麻醉药注射在含有颈交感神经节的硫松结缔组织内而阻滞支配头面颈部、上肢及上胸部交感神经的方法。对它的机制、临床应用、操作手法等已进行了大量研究和临床实验,目前的研究表明SGB可明显扩张脑血管,增加脑血流速度,改善兔全脑缺血-再灌注后ET与CGRP平衡失调及神经细胞热休克蛋白70的过度表达[9-10],对脑缺血-再灌注脑损伤有较好的保护作用。颈交感神经阻滞可降低大鼠术后血清S100及NSE的浓度,改善老年大鼠术后认知功能[11-12]。本研究旨在探讨颈交感神经阻滞能否通过降低炎症反应从而保护糖尿病大鼠脑线粒体的功能。
炎症是机体对损伤因子所发生的防御反应。激活的单核巨噬细胞等免疫细胞合成、分泌的多种促炎因子,如肿瘤坏死因子(tumor necrosis factor,TNF)、白细胞介素-1(interleukin-1,IL-1) 等,有利于清除外来病原体及坏死组织,促进组织修复及创口愈合,是机体防御机制的重要因素。但是由于各种原因,机体的自身调节功能失衡,产生过量的促炎因子,引起瀑布样炎症反应,导致炎症反应失控,引发全身炎症反应综合征(systemic inflammatory response syndrome,SIRS)甚至多器官功能障碍综合征。交感神经作用的受体为肾上腺素能受体,分为α和β两种受体,广泛分布于人体的组织和器官。目前的研究表明,Kupffer细胞β肾上腺素能受体表达上调,导致细胞内cAMP含量上升,并与炎症抑制相关。Weiss等的研究结果表明,TNFα的表达和星形胶质细胞的反应有相关性。对其他单核巨噬细胞的研究发现,应用β肾上腺素能受体激动剂可显著抑制TNF-α、IL-6等促炎因子的释放[13-14]。本研究观察到糖尿病组和阻滞组IL-6、TNF-α表达均高于对照组,但阻滞组又明显低于糖尿病组。表明糖尿病大鼠颅内炎症反应增加,颈交感神经阻滞可抑制炎症反应。
线粒体是膜性细胞器,炎症反应容易导致膜脂质氧化,损伤线粒体。目前的研究表明,在糖尿病脑病的发生发展中,由于高血糖、氧化应激损伤神经细胞线粒体,线粒体功能异常伴随自噬活化是糖尿病脑病脑损伤的病理变化[15]。本研究结果显示,糖尿病组和阻滞组海马线粒体呼吸功能均较对照组明显下降,主要表现为R3和RCR值显著降低;但阻滞组R3和RCR明显高于糖尿病组。表明糖尿病大鼠脑线粒体功能受损,颈交感神经阻滞可改善糖尿病大鼠的线粒体功能,其机制可能与颈交感神经阻滞可抑制炎症反应有关。本研究观察到水迷宫行为学测试造模后12周糖尿病组、阻滞组逃避潜伏期较造模前明显延长、穿越平台的次数较造模前明显减少。与糖尿病组比较,阻滞组大鼠逃避潜伏期缩短、穿越平台的次数增加,但差异无统计学意义,可能与本研究样本量较少有关。
综上所述,本研究结果表明,颈交感神经阻滞可改善糖尿病大鼠脑线粒体功能,其机制可能与其下调IL-6、TNF-α抑制炎症反应减轻脑损伤有关,但对认知功能障碍无明显改善,可能与本研究样本量较少有关,但其在临床中的运用仍需更多的基础研究来证实。
| [1] | RAMOS-RODRIGNEZ J J, ORTIZ O, JIMENEZ-PALOMARES M, et al. Differential central pathology and cognitive impairment in prediabetic and diabetic mice[J]. Psychoneuroendocrinology, 2013, 38(11): 2462–2475. DOI:10.1016/j.psyneuen.2013.05.010 |
| [2] | SONNEN J A, LN E B, BRICKELL K, et al. Different patterns of cerebral injury in dementia with or without diabetes[J]. Arch Neurol, 2009, 66(3): 315–322. DOI:10.1001/archneurol.2008.579 |
| [3] |
庄丽英, 张志瑁. 神经炎症与阿尔茨海默病[J].
中华行为医学与脑科学杂志, 2012, 21(7): 664–665.
ZHUANG L Y, ZHANG Z M. Nerve inflammation and Alzheimer's disease[J]. Chin J Behav Med Brain Sci, 2012, 21(7): 664–665. DOI:10.3760/cma.j.issn.1674-6554.2012.07.030 |
| [4] | SONNEN J A, LARSON E B, BRICKELL K, et al. Different patterns of cerebral injury in dementia with or without diabetes[J]. Arch Neurol, 2009, 66(3): 315–322. DOI:10.1001/archneurol.2008.579 |
| [5] | REDDY P H, REDDY T P. Mitochondria as a therapeutic target for aging and neurodegenerative diseases[J]. Curr Alzheimer Res, 2011, 8(4): 393–409. DOI:10.2174/156720511795745401 |
| [6] | DINEL A L, ANDRE C, AUBERT A, et al. Cognitive and emotional alterations are related to hippocampal inflammation in a mouse model of metabolic syndrome[J]. Plos One, 2011, 6(9): e24325. DOI:10.1371/journal.pone.0024325 |
| [7] | YU N, WANG S, WANG P, et al. The calcium uniporter regulates the permeability transition pore in isolated cortical mitochondria[J]. Neural Regen Res, 2012, 7(2): 109–113. DOI:10.3969/j.issn.1673-5374.2012.02.005 |
| [8] |
吴晶, 张明强, 贾敏, 等. 线粒体抗氧化肽对脓毒性脑病小鼠认知功能和线粒体功能的影响[J].
临床麻醉学杂志, 2014, 8(30): 788–791.
WU J, ZHANG M Q, JIA M, et al. Effects of mitochondrial-targeted peptide on cognitive and mitochondrial functions in a mouse model of sepsis-associated encephalopathy[J]. J Clin Anesthesiol, 2014, 8(30): 788–791. |
| [9] | SARIOǦLU Y, UTKAN T, AKGVN M, et al. Effects of deferoxamine and sympathectomy on endothelin-1-induced contraction and acetylcholine-induced relaxation following subarachnoid hemorrhage in carotid artery[J]. Gen Pharmacol, 1997, 28(1): 145–151. DOI:10.1016/S0306-3623(96)00154-1 |
| [10] | TREGGIARI M M, ROMAND J A, MARTIN J B, et al. Cervical sympathetic block to reverse delayed ischemic neurological deficits after aneurismal subarachnoid hemorrhage[J]. Stroke, 2003, 34(4): 961–967. DOI:10.1161/01.STR.0000060893.72098.80 |
| [11] | HE C J, LIU G D, NIE H X, et al. Effect of cervical sympathetic block on cerebral vasospasm after subarachnoid hemorrhage in rabbits[J]. Acta Cir Bra, 2013, 28(1): 1–11. DOI:10.1590/S0102-86502013000100001 |
| [12] | LIU G D, HE C J. Stellate ganglion block promotes recovery of Bell's palsy in patients with diabetes mellitus[J]. Acta Otolaryngol, 2014, 134(6): 652–655. DOI:10.3109/00016489.2014.880794 |
| [13] | ZEINSTRA E, WILCZAK N, DE KEYSER J. [3H]dihydroalprenolol binding to beta adrenergic receptors in multiple sclerosis brain[J]. Neurosci Lett, 2000, 289(1): 75–77. DOI:10.1016/S0304-3940(00)01254-4 |
| [14] | MORIN D, SAPENA R, ZINI R, et al. Characterization of beta-adrenergic receptors of freshly isolated astrocytes and neurons from rat brain[J]. Life Sci, 1997, 60(4-5): 315–324. |
| [15] | RAINS J L, JAIN S K. Oxidative stress, insulin signaling, and diabetes[J]. Free Radic Biol Med, 2011, 50(5): 567–575. DOI:10.1016/j.freeradbiomed.2010.12.006 |



