肝癌是全球癌症相关死亡排名第二的肿瘤[1],主要包括肝细胞癌(hepatocellular carcinoma,HCC)和胆管细胞癌。其中主要为HCC,在全球常见恶性肿瘤中位居第五[2]。HCC主要病因为HBV与HCV感染[3],在中国,由上述两种病毒感染所致的HCC占全部HCC的86%[4]。肝切除术是目前治疗HCC最主要的方法,根据HCC分期的不同,其5年生存率为38%~61%[5]。但由于早期诊断的困难,只有30%~40%的患者能获得根治性治疗[6]。传统的肿瘤标记物甲胎蛋白(α-fetoprotein,AFP)的检测,常规用于临床HCC的筛查。现有研究证明,AFP在诊断HCC时,灵敏度为39%~65%,特异度为76%~93%,大约1/3早期HCC患者不能得到诊断[2]。因此寻找特异性的早期HCC诊断标志物特别重要。
miRNA是长19~22个核苷酸内源性表达的单链非编码RNA。其广泛存在于真核生物中,主要与mRNA的3′UTR端结合而调节靶基因的表达,从而调节细胞的增殖、分化、凋亡、浸润和迁移[7]。现在观点认为miRNA能在循环中稳定地表达,能抵制核糖核酸酶A的降解,甚至在pH值为1和13的溶液中保持稳定[8]。基于此类研究的发现,国内外学者开始对HCC患者循环中的miRNA的诊断价值进行论证,并取得了一定的成绩,为HCC诊断开辟了新的研究方向。Qi等[9]指出,miR-122在HCC患者血清中表达水平明显高于健康对照者,能作为HCC肿瘤标记物区分两组人群。Tomimaru等[10]发现循环miR-21能够作为HCC肿瘤标记物,从而把HCC与健康人群、慢性肝炎患者区别。这些研究为寻找HCC新的肿瘤标记物提供了线索。本实验旨在探索具有诊断潜力的HCC相关miRNA肿瘤标记物,并验证其与临床参数的相关性。
1 资料与方法 1.1 实验设计和一般资料分为4个阶段。第一阶段:组织样本候选miRNA筛查。取肝癌切除术患者癌组织和癌旁非肿瘤组织进行miRNA芯片检测,筛查异常表达的miRNA,选择高表达的miRNA作为候选miRNA进行下一步实验。第二阶段:目的miRNA的确定。取术前及术后7~10 d HCC患者血浆进行定量RT-PCR,术后表达量下降的候选miRNA定为目的miRNA进行下一步研究。第三阶段:目的miRNA的验证。所有HCC患者术前血浆、肝硬化(hepatocirrhosis,CIR)及健康对照者血浆进行定量逆转录-聚合酶链反应(quantitative reverse transcription-polymerase chain reaction,qRT-PCR),检验目的miRNA在3组人群血浆中的表达差异。第四阶段:诊断价值验证及相关性分析。绘制受试者工作曲线(receiver operating characteristic curve,ROC),对目的miRNA的诊断价值进行评价,并分析目的miRNA与临床参数之间的相关性。
所有组织和血液样本来自本院。组织样本于2010年6月收集并送公司检测,血液样本于2014年 1-6月收集,包括肝细胞癌患者39例,CIR患者32例,健康对照者25例。所有肝细胞肝癌经病理证实,组织和血液样本的使用取得患者同意。
1.2 组织和血浆肝细胞癌肿瘤组织和癌旁组织来源于肝癌切除术患者,为分离血浆,2 mL外周血经EDTA抗凝后立即行 4 ℃,2 000 r/min离心10 min,上清液转入新EP管,4 ℃,13 000 r/min离心8 min,收集上清液,-80 ℃贮存。
1.3 组织miRNA芯片癌组织及癌旁正常组织送重庆威斯腾生物技术服务中心进行miRNA芯片检测。
1.4 血浆RNA提取使用TRIzol LS (Invitrogen)试剂提取血浆中总RNA,具体提取步骤根据试剂说明进行。RNA纯度和 浓度用NanodropND2000分光光度计(Thermo Scientific,Worcester,MA,USA)检测。D(260)/D(280)为1.9~2.1,总RNA浓度为25~120 ng/μL。
1.5 qRT-PCR血浆miRNA的逆转录和定量检测采用ALL-in-OneTM miRNA qRT-PCR Detection Kit(GeneCopoeia公司,广州)。miRNA引物购自GeneCopoeia公司(广州)。25 μL逆转录体系包括总RNA:10 μL,Poly A 聚合酶(2.5 U/μL):1 μL,RTase Mix:1 μL,5×PAP/RT Buffer:5 μL,dd H2O:8 μL。逆转录执行条件:37 ℃ 孵育60 min,85 ℃孵育5 min使酶灭活。定量PCR采 用20 μL体系[逆转录产物即cDNA: 2 μL,2×ALL-in-One qPCR Mix: 10 μL,ALL-in-OneTM miRNA引物:2 μL,Universal Adaptor PCR 引物(稀释至2 μmol/μL): 2 μL ,ddH2O: 4 μL],定量PCR条件:95 ℃ 10 s,40个循环:95 ℃ 10 s,60 ℃ 20 s,72 ℃ 10 s。每个孔重复3次,取平均Ct值。在循环miRNA的研究中,目前没有公认的内参,根据文献[9, 10]选择U6 RNA和miR-16进行内参筛选。由于U6 RNA在多数 样本中无法检测,miR-16在所有样本中稳定表达,最终选择miR-16作为内参。miRNA的表达水平用2-ΔΔCt值表 示[11],ΔCt=CtmiRNA-CtmiR-16,ΔΔCt=ΔCtmiRNA(样本A)- ΔCtmiRNA(样本B)。
1.6 统计学分析组织样本miRNA表达差异采用分层汇聚和相关分析,统计数据由重庆威斯腾生物技术服务中心提供。血浆miRNA表达水平差异、与临床参数的相关性及诊断价值评估采用SPSS 17.0统计软件进行统计分析。术前和术后3种血浆miRNA表达差异采用秩和检验(Mann-Whitney test)。miRNA与临床病例参数之间的相关性,先按临床参数进行分组,秩和检验分析组间表达水平的差异,Spearman秩相关性分析检验其相关性。GraphPad Prism 5绘制ROC曲线和计算曲线下面积(area under the curve,AUC)评估目的miRNA的诊断价值,计算Youden指数(Youden’s index)选择最佳的界值(cutoff value)。
2 结果 2.1 候选miRNA的筛选组织miRNA芯片结果显示:癌组织中40种miRNA表达量增加(>2倍);52种miRNA表达量明显下降(<0.5倍)。表 1列举了部分肿瘤组织中异常表达的miRNAs,选取癌组织中表达量最高的2种miRNAs即miR-15b-5p、miR-338-5p进行研究。miR-21-5p在HCC中作用机制研究较多,且在肝癌切除术后表达水平下降,增加miR-21-5p作为候选miRNA进行下一步研究有利于实验的质量控制。
| miRNA | C/N | C | N |
| 癌组织中表达量增加 | |||
| miR-15b-5p | 2.541 2 | 1.865 7 | 0.734 2 |
| miR-338-5p | 13.792 4 | 2.202 5 | 0.159 7 |
| miR-452 | 2.002 7 | 0.347 1 | 0.173 0 |
| miR-25 | 2.024 3 | 0.642 7 | 0.317 4 |
| miR-21* | 2.121 9 | 0.359 5 | 0.169 4 |
| 癌组织中表达量减少 | |||
| miR-519e | 0.216 0 | 1.859 5 | 8.609 5 |
| miR-130a | 0.340 4 | 0.460 7 | 1.353 5 |
| miR-138-2* | 0.418 8 | 0.093 0 | 0.222 0 |
| miR-19b | 0.477 4 | 0.892 6 | 1.869 5 |
| miR-1201 | 0.393 3 | 0.157 0 | 0.399 2 |
*:暂未统一命名
HCC患者术前、术后血浆qRT-PCR结果显示:miR-15-5p(P=0.006)、miR-21-5p(P=0.011)、miR-338-5p(P=0.002)术后明显降低。上述3种miRNA在肿瘤组织和血浆中均高表达,且术后表达水平明显下降,提示3种候选miRNA均为肿瘤来源,定为目的miRNA进行下一步实验(表 2)。
| 时间 | miR-15b-5p | miR-21-5p | miR-338-5p |
| 术前 | 2.05(1.26,3.60) | 2.79(1.83,13.97) | 3.21(2.10,8.73) |
| 术后 | 1.00(0.62,1.00) | 1.00(1.00,1.43) | 1.00(1.00,1.19) |
| P值 | 0.006 | 0.011 | 0.002 |
使用qRT-PCR检测剩余26例HCC患者、32例CIR患者和25例健康对照者血浆中目的miRNA的表 达水平,并把术前13例HCC患者样本纳入统计,共39例 HCC样本(样本总量为96个)。结果见表 3,图 1。
| 组别 | 例数 | miR-15b-5p | miR-21-5p | miR-338-5p |
| HCC | 39 | 1.45(1.00,2.87) | 2.56(1.27,7.65) | 2.92(1.36,8.27) |
| CIR | 32 | 1.00(0.88,1.09)a | 1.00(0.89,1.47)a | 1.00(0.82,1.63)a |
| HC | 25 | 0.41(0.24,0.88)ac | 0.53(0.19,1.13)ab | 0.34(0.09,0.88)ac |
a:P<0.01,与HCC比较;b:P<0.05,c:P<0.01,与CIR比较
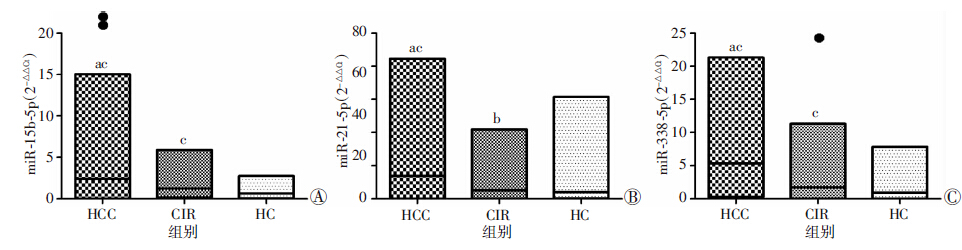 |
| a:P<0.01,与CIR组比较;b: P<0.05,c:P<0.01,与HC组比较( ● :示离群值)图 1 miR-15b-5p、miR-21-5p、miR-338-5p在HCC、CIR、HC血浆中的表达差异 |
由表 3和图 1可见,HCC组miR-15b-5p、miR-21-5p、miR-338-5p表达水平明显高于CIR组(P<0.01)和HC组(P<0.01)。同时可以看出CIR组miR-15b-5p、miR-21-5p、miR-338-5p表达水平也明显高于HC组(P<0.01),由图 1我们还可以看出HCC组miR-15b-5p、CIR组miR-338-5p存在离群值,且表达量很高,暂不做进一步研究。
2.4 ROC曲线分析为了评估miR-15b-5p、miR-21-5p、miR-338-5p作为诊断HCC的肿瘤标记物的价值,我们分3种模式(HCC和CIR区别、HCC和HC区别、HCC区别CIR和HC的联合)绘制ROC曲线,分别计算其AUC,并根据最大Youden指数选择作为HCC肿瘤标记物的界值。我们发现3种miRNA在区别HCC患者、CIR患者和健康人群时,都有较好的诊断价值。为了验证3种miRNA在所有样本中鉴别HCC患者的能力,我们把所有样本同时纳入绘制ROC曲线(第3种模式),结果显示,miR-338-5p≥1.743时,把HCC患者从总的96个样本中区分出的灵敏度、特异度、AUC较高,表现出较高的诊断价值。同样的方法进一步对miR-21-5p进行分析,也表现出较好的价值,而miR-15b-5p的AUC相对较小。总的来说,3种miRNA在区别HCC患者和健康对照人群所表现出的AUC大于区别HCC和CIR所表现出的AUC。结果见表 4,图 2。
| miRNA | HCC和CIR区别 | HCC和HC区别 | HCC区别CIR和 HC的联合 |
| miR-15b-5p | 1.025,0.680 (66.67%,75.00%) | 0.807,0.856 (87.18%,76.00%) | 1.025,0.758 (66.67%,78.95%) |
| miR-21-5p | 1.353,0.756 (74.36%,75.00%) | 0.772,0.843 (94.87%,64.00%) | 1.393,0.794 (74.36%,78.95%) |
| miR-338-5p | 2.270,0.762 (64.10%,87.50%) | 0.981,0.905 (89.74%,84.00%) | 1.743,0.825 (69.23%,85.96%) |
 |
| A~C:miR-15b-5p作为标记物在把HCC从CIR、HC、CIR+HC区分出来的表现;D~F:miR-21-5p作为标记物在把HCC从CIR、HC、CIR+HC区分出来的表现;G~I:miR-338-5p作为标记物在把HCC从CIR、HC、CIR+HC区分出来的表现图 2 miR-15b-5p、miR-21-5p、miR-338-5p作为HCC肿瘤标记物在3种模式中的ROC曲线表现 |
由于miR-21-5p和miR-338-5p总的AUC较大,我们分两种模式对其灵敏度和特异度进行探讨。当miR-21-5p≥1.393 0,miR-338-5p≥1.743 0两者满足其一即诊断为HCC时,灵敏度为79.49%,特异度为75.44%,当两者都满足才能诊断为HCC时,灵敏度为64.10%,特异度为92.98%。
2.5 miRNA血浆表达水平与临床参数相关性分析进一步与临床参数结合分析结果显示,AFP<200 ng/mL 的HCC患者中,以miR-15b-5p≥1.025、miR-21-5p≥1.393、miR-338-5p≥1.743作为诊断标准,其灵敏度分别为72.73%、86.36%、81.82%。而纳入实验的HCC患者,若以AFP≥200 ng/mL作为诊 断标准,灵敏度仅为43.59%,与AFP灵敏度为39%~65%相符合。我们还可以看出miR-21-5p、miR-338-5p 灵敏度较高,十分有利于AFP不能发现的早期HCC患者的诊断。
进一步按临床参数分组对3种miRNA表达水平进行验证。表 5显示:在AFP<200 ng/mL的患者血浆中,miR-338-5p的表达水平较高(P=0.036)。但3种 miRNA的表达水平与肿瘤直径、胆红素、转氨酶水平及HBsAg是否阳性无关。进一步Spearman秩相关性分析结果显示,miR-338-5p表达量与AFP水平呈负相关(r=-0.344,P=0.032)。鉴于本实验HCC样本中性别、年龄及Child-Pugh分级所表现出的人数差异过大,不做相关性分析。
| 参数 | 人数(个) | P值 | ||||
| HCC | CIR | HC | miR-15b-5p | miR-21-5p | miR-338-5p | |
| 性别 | ||||||
| 男 | 36 | 20 | 12 | |||
| 女 | 3 | 12 | 13 | |||
| 年龄(岁) | ||||||
| ≥60 | 6 | 5 | 0 | |||
| <60 | 33 | 27 | 25 | |||
| AFP(ng/mL) | 0.436 | 0.061 | 0.036 | |||
| ≥200 | 17 | |||||
| <200 | 22 | |||||
| ALT(U/L) | 0.855 | 0.144 | 0.605 | |||
| ≥40 | 27 | |||||
| <40 | 12 | |||||
| AST(U/L) | 0.803 | 0.379 | 0.907 | |||
| ≥40 | 25 | |||||
| <40 | 14 | |||||
| TBiL(μmol/L) | 0.725 | 0.800 | 0.673 | |||
| ≥17.1 | 19 | |||||
| <17.1 | 20 | |||||
| HBsAg | ||||||
| 阳性 | 22 | |||||
| 阴性 | 7 | |||||
| Child-Pugh分级 | ||||||
| A | 36 | |||||
| B | 3 | |||||
| C | 0 | |||||
| 肿瘤直径(cm) | 0.398 | 0.248 | 0.324 | |||
| ≥5 | 21 | |||||
| <5 | 18 | |||||
循环miRNA能够作为诊断HCC的标记物,即使在AFP<200 ng/mL的样本中,也能取得比较满意的诊断价值。本研究参考了国际经典的miRNA筛查途径,并把实验分为4个阶段。随着研究的深入,我们发现如下现象:①miR-338-5p在AFP<200 ng/mL的HCC患者血浆中表达水平较高,十分有利于AFP阴性的HCC患者的早期诊断,因为肝癌细胞来源的AFP早期水平较低,miR-338-5p在此阶段的高水平恰好弥补AFP在此阶段诊断的不足。②miR-15b-5p、miR-21-5p、miR-338-5p在鉴别HCC患者和健康对照者表现出的AUC明显大于鉴别HCC和肝硬化时的AUC,可能提示这3种miRNA在正常肝脏向肝硬化转变,以及肝硬化向HCC转变的过程中发挥作用。
越来越多的研究表明,循环miRNA有作为HCC肿瘤标记物的潜力;Li等[12]指出,循环miR-18a作为HBV相关HCC肿瘤标记物有潜在价值;为寻求更加简便的诊断标记物,Abdalla等[13]对尿液中的miRNA进行筛查,发现了miR-618和miR-650,但总的诊断价值不高。除了上述与HCC诊断相关的循环miRNA外,近期的研究还证实miR-221、miR-885-5p、miR-122a均有作为HCC肿瘤标记物的潜质[14, 15, 16]。
关于miR-338-5p和HCC关系的研究尚少见报道,对miR-15b-5p的文献报道也有限。Dai等[17]报道,miR-15b通过肝细胞核因子1α调节乙肝病毒的复制。Wu等[18]指出,乙肝病毒X蛋白抑制miR-15b的表达,从而增强肝细胞癌的增殖能力。也有研究指出miR-15b在肝癌组织中高表达,并在细胞有丝分裂周期中发挥调节作用[19]。关于miR-21的报道很多,Xu 等[20]发现miR-21的靶点为丝裂原活化蛋白激酶-激酶3(mitogen-activated protein kinase-kinase 3,MAP2K3),miR-21通过抑制其表达,促进肝癌细胞增殖。Tomimaru等[10]和Xu等[21]认为循环miR-21可以作为肝癌或者慢性肝炎的标记物。miR-15b-5p和miR-21-5p在HCC癌组织中高表达已经被证实,提示其为肿瘤组织来源。前者可以调节细胞周期的进程,后者影响肿瘤的迁移和浸润能力,均在肿瘤的发生、发展过程中发挥作用。虽然miR-338-5p在HCC形成过程中的作用不清,但是我们发现miR-338-5p为肿瘤组织来源的证据。这3种miRNA在HCC发展过程中,由癌组织进入血液循环,并稳定地表达,为我们检测循环miRNA筛查HCC提供了可能。
现有研究也证实循环miRNA有作为甲状腺癌[22]、乳腺癌[23]、胰腺癌[24]、肺癌[25]、胃癌[26]等肿瘤标记物的可能。
本研究结果证实miR-15b-5p、miR-21-5p具有作为肿瘤标记物潜力的同时,发现miR-338-5p也具有作为HCC肿瘤标记物的潜力,并且miR-21-5p和miR-338-5p联合作为HCC肿瘤标记物,具有很高的特异性。即使在AFP判定为阴性的情况下,3种miRNA都具有较高的灵敏度。我们纳入研究的HCC患者血浆样本为39例,诊断价值评价结果及相关数据虽然显示出了一定的诊断价值趋势,但有待更大样本的实验证实。本研究丰富了寻找无创的能弥补AFP不足的HCC肿瘤标记物的实例,为进一步更大范围和规模的研究提供了参考。
志谢 感谢重庆市妇产科重点实验室及于廷和教授对本实验的支持
| [1] | Lozano R,Naghavi M,Foreman K,et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010:a systematic analysis for the Global Burden of Disease Study 2010[J]. Lancet,2012,380(9859): 2095-2128. |
| [2] | Morise Z,Kawabe N,Tomishige H,et al. Recent advances in the surgical treatment of hepatocellular carcinoma[J]. World J Gastroenterol,2014,20(39): 14381-14392. |
| [3] | Eskens F A,van-Erpecum K J,de-Jong K P,et al. Hepatocellular carcinoma:Dutch guideline for surveillance, diagnosis and therapy[J]. Neth J Med,2014,72(6): 299-304. |
| [4] | de-Martel C,Ferlay J,Franceschi S,et al. Global burden of cancers attributable to infections in 2008:a review and synthetic analysis[J]. Lancet Oncol,2012,13(6): 607-615. |
| [5] | Torzilli G,Belghiti J,Kokudo N,et al. A snapshot of the effective indications and results of surgery for hepatocellular carcinoma in tertiary referral centers:is it adherent to the EASL/AASLD recommendations?:an observational study of the HCC East-West study group[J]. Ann Surg,2013,257(5): 929-937. |
| [6] | Zhou J,Yu L,Gao X,et al. Plasma microRNA panel to diagnose hepatitis B virus-related hepatocellular carcinoma[J]. J Clin Oncol,2011,29(36): 4781-4788. |
| [7] | Zhu Z,Zhang X,Wang G,et al. Role of MicroRNAs in Hepatocellular Carcinoma[J]. Hepat Mon,2014,14(8): e18672. |
| [8] | Chen X,Ba Y,Ma L,et al. Characterization of microRNAs in serum:a novel class of biomarkers for diagnosis of cancer and other diseases[J]. Cell Res,2008,18(10): 997-1006. |
| [9] | Qi P,Cheng S Q,Wang H,et al. Serum microRNAs as biomarkers for hepatocellular carcinoma in Chinese patients with chronic hepatitis B virus infection[J]. PLoS One,2011,6(12): e28486. |
| [10] | Tomimaru Y,Eguchi H,Nagano H,et al. Circulating microRNA-21 as a novel biomarker for hepatocellular carcinoma[J]. J Hepatol,2012,56(1): 167-175. |
| [11] | Karakatsanis A,Papaconstantinou I,Gazouli M,et al. Expression of microRNAs,miR-21,miR-31,miR-122,miR-145,miR-146a,miR-200c,miR-221,miR-222,and miR-223 in patients with hepatocellular carcinoma or intrahepatic cholangiocarcinoma and its prognostic significance[J]. Mol Carcinog,2013,52(4):297-303. |
| [12] | Li L,Guo Z,Wang J,et al. Serum miR-18a:a potential marker for hepatitis B virus-related hepatocellular carcinoma screening[J]. Dig Dis Sci,2012,57(11): 2910-2916. |
| [13] | Abdalla M A,Haj-Ahmad Y. Promising Candidate Urinary MicroRNA Biomarkers for the Early Detection of Hepatocellular Carcinoma among High-Risk Hepatitis C Virus Egyptian Patients[J]. J Cancer,2012,3: 19-31. |
| [14] | Li J,Wang Y,Yu W,et al. Expression of serum miR-221 in human hepatocellular carcinoma and its prognostic significance[J]. Biochem Biophys Res Commun,2011,406(1): 70-73. |
| [15] | Gui J,Tian Y,Wen X. et al. Serum microRNA characterization identifies miR-885-5p as a potential marker for detecting liver pathologies[J]. Clin Sci (Lond),2011,120(5): 183-193. |
| [16] | Luo J,Chen M,Huang H,et al. Circulating microRNA-122a as a diagnostic marker for hepatocellular carcinoma[J]. Onco Targets Ther,2013,6: 577-583. |
| [17] | Dai X,Zhang W,Zhang H,et al. Modulation of HBV replication by microRNA-15b through targeting hepatocyte nuclear factor 1α[J]. Nucleic Acids Res,2014,42(10): 6578-6590. |
| [18] | Wu C S,Yen C J,Chou R H,et al. Downregulation of microRNA-15b by hepatitis B virus X enhances hepatocellular carcinoma proliferation via fucosyltransferase 2-induced Globo H expression[J]. Int J Cancer,2014,134(7): 1638-1647. |
| [19] | Lin L,Lin Y,Jin Y,et al. Microarray analysis of microRNA expression in liver cancer tissues and normal control[J]. Gene,2013,523(2): 158-160. |
| [20] | Xu G,Zhang Y,Wei J,et al. MicroRNA-21 promotes hepatocellular carcinoma HepG2 cell proliferation through repression of mitogen-activated protein kinase-kinase 3[J]. BMC Cancer,2013,13: 469. |
| [21] | Xu J,Wu C,Che X,et al. Circulating microRNAs,miR-21,miR-122,and miR-223,in patients with hepatocellular carcinoma or chronic hepatitis[J]. Mol Carcinog,2011,50(2): 136-142. |
| [22] | Cantara S,Pilli T,Sebastiani G,et al. Circulating miRNA95 and miRNA190 are sensitive markers for the differential diagnosis of thyroid nodules in a Caucasian population[J]. J Clin Endocrinol Metab,2014,99(11): 4190-4198. |
| [23] | Shen J,Hu Q,Schrauder M,et al. Circulating miR-148b and miR-133a as biomarkers for breast cancer detection[J]. Oncotarget,2014,5(14): 5284-5294. |
| [24] | Wang J,Raimondo M,Guha S,et al. Circulating micro RNAs in Pancreatic Juice as Candidate Biomarkers of Pancreatic Cancer[J]. J Cancer,2014,5(8): 696-705. |
| [25] | Ulivi P,Zoli W. miRNAs as non-invasive biomarkers forlung cancer diagnosis[J]. Molecules,2014,19(6): 8220-8237. |
| [26] | Liu L,Wang S,Cao X,et al. Diagnostic value of circulating microRNAs for gastric cancer in Asian populations:a meta-analysis[J]. Tumour Biol,2014,35(12): 11995-12004. |



