心力衰竭(heart failure,HF)是最常见、危害最大的心血管病之一,为多种心血管疾病终末期表现。HF发生、进展过程中交感神经功能异常发挥重要作用。HF时交感神经变化主要表现为神经活性(sympathetic nerve activity,SNA)增加、神经密度降低、再摄取(reuptake)功能下降[1, 2]。有研究观察到HF时心肌神经生长因子(nerve growth factor,NGF)及受体表达(TrkA)降低,认为与心脏交感神经支配密度/再摄取降低有关[2, 3]。还有研究显示,心脏压力超负荷(pressure overload,POL)后心肌交感神经密度增加,但交感神经递质转运蛋白(norepinephrine transporter,NET)再摄取功能降低[2, 4]。目前对HF时心脏交感神经重塑机制还不完全清楚。心脏超负荷导致心脏重构、交感神经激活,是导致HF最重要病因之一[1, 2, 5]。本实验通过腹主动脉缩窄大鼠模型,观察POL后心脏交感神经NET、心肌NGF蛋白及受体表达以及神经生长相关蛋白(growth associated protein 43,GAP43)表达变化,并讨论其意义及机制。 1 材料与方法 1.1 实验动物
40只7~8周龄雄性Wistar大鼠,体质量(156±10)g,购自中国人民解放军军事医学科学院实验动物中心。室温18~25 ℃、普通饲料、自由饮水。将大鼠分为长期压力超负荷(POL)组(n=30)和假手术组(n=10)。 1.2 主要仪器和试剂
心脏彩色超声诊断仪:GE Vivid 7 Ultrasound System(Alliance Medical Systems公司,USA),ABI Prism 7000型荧光定量PCR仪(Applied Biosystems),80i荧光显微镜(日本Nikon公司)。主要试剂:Trizol试剂(Invitrogen 公司),NGF多克隆抗体(兔抗大鼠,美国Santa Cruz公司),TRITC标记羊抗兔IgG(美国Zymed公司)。 1.3 方法 1.3.1 腹主动脉缩窄大鼠心脏POL模型建立
大鼠经0.4%戊巴比妥钠(40 mg/kg)腹腔麻醉后,仰卧固定,常规消毒,开腹,分离肾动脉上腹主动脉,于双肾动脉上方0.5 cm处用7号针头与腹主动脉平行放置,共同结扎后抽出针头,即形成腹主动脉部分狭窄。假手术组仅分离腹主动脉,不结扎[6]。缩窄后8周用超声心动图检测其心功能指标。 1.3.2 超声心动图检测
0.4%戊巴比妥钠(10 mL/kg)腹腔麻醉,仰卧固定,胸部涂超声耦合剂,用12 MHz超声探头行心脏超声检测。 1.3.3 取材与标本处理
超声检测后,称量并处死动物。立即摘取双侧心脏交感神经节(颈中-星状神经节复合体,middle cervical-stellate ganglion complex,MC-SGC)[7],迅速置于液氮中速冻后,置于-80 ℃冰箱保存备测。摘取心脏,用电子天平称量右心室、左心室,计算左心室质量/体重即心室质量指数(left ventricular mass index,LVMI)。 1.3.4 免疫荧光检测心肌NGF表达
取左室标本,OCT包埋,置于冰冻切片机,连续8 μm厚度制成横断切片,每个标本按3个不同部位各取10张切片。用NGF多克隆抗体以及TRITC标记的羊抗兔IgG标记NGF,于80i型荧光显微镜下观察检测心肌NGF表达。以PBS液替代双抗为阴性对照。用Image Pro Plus 4.5图像分析软件进行图像分析[6]。 1.3.5 实时荧光定量PCR检测NET、GAP43、TrKA mRNA表达
按照TRIzol试剂盒操作手册,提取MC-SGC及心肌中总RNA。鉴定RNA完整性,并定量。通过SuperscriptⅡ反转录酶反转录获得cDNA。按GenBank中大鼠NET、GAP43、TrKA、β-actin序列,利用ABI Prism 7300自带的引物设计软件(Primer Express)设计以下配对引物: NET正义链:5′-TCCATTCTCTTTGCCGTGCT -3′ ,反义链:5′-CCTGGCTTAAACCCCATCATC-3′ 。GAP43正义链:5′-TGTACCCCGGTTTTTTGATCTG-3′ ,反义链:5′-CAGAACGGAACATTGCACACA-3′。TrKA正义链:5′-ATCCTCTACCGCAAGTTCAGCA-3′,反义链:5′-ATCGCCTCAGTGTTGGAGA-GCT-3′。β-actin 正义链:5′-TCTGTGTGGATTGGTGGCTCT-3′ ,反义链:5′-AGAAGCATTTGCGGTGCAC-3′(引物由上海博亚生物技术公司合成)[6]。
Real-time荧光定量PCR反应在ABI Prism 7000型荧光定量PCR仪中进行。50 ℃孵育2 min,然后95 ℃,10 min;接着进行45个循环,95 ℃,15 s,59 ℃,1 min,72 ℃,20 s。每个样本重复3次。通过参数设定以及ABI PRISM Sequence Detection software处理分析,得到不同样本相对于不同基因Ct值,通过检测每个样品管家基因(β-actin),将待测基因归一化。得到 ΔCt=Ct待测基因-Ct管家基因(β-actin),再转换为原始模板浓度= 2-ΔCt,得出检测目标原始模板相对表达量。 1.4 统计学分析
采用SPSS 16.0统计软件,实验结果以x±s表示,均数之间的比较用成组双样本t检验。 2 结果 2.1 2组大鼠心脏功能评价
与假手术组比较,POL大鼠可见左心室肥厚,心腔扩大。超声心动图检测示POL大鼠心室间隔舒张期厚度(interventricularseptal thickness during diastole,IVSd)、收缩期心室间隔厚度(interventricularseptal thickness during systole,LVSs)、舒张期左室后壁厚度(diastolic LV posterior wall thickness,LVPWd)、射血分数(ejection fraction,EF)、左室内膜缩短率(LV endocardial fractional shortening,FS%)、LVMI显著升高(P<0.05),表明POL诱导大鼠出现心肌肥厚,心功能代偿(表 1)。
| 组别 | n | 室间隔舒张期厚度(cm) | 收缩期心室间隔厚度(cm) | 舒张期左室后壁厚度(cm) | 射血分数(%) | 左室内膜缩短率(%) | 心室质量指数(mg/g) |
| 假手术组 | 10 | 0.175±0.044 | 0.285±0.062 | 0.160±0.050 | 0.684±0.038 | 0.333±0.028 | 2.039±0.102 |
| POL组 | 30 | 0.280±0.042a | 0.369±0.047a | 0.244±0.054a | 0.765±0.059a | 0.399±0.049a | 2.716±0.129a |
| a:P<0.05,与假手术组比较 | |||||||
与假手术组比较,POL大鼠心肌NGF蛋白表达增加(P<0.05,图 1)。
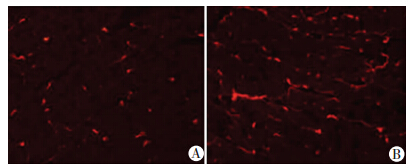 |
| A:假手术组;B:POL组 图 1 免疫荧光染色观察2组大鼠心肌NGF蛋白表达(荧光显微镜 ×200) |
实时荧光定量PCR结果显示,与假手术组比较,POL大鼠NET、GAP43 mRNA表达水平无变化(P>0.05),同时检测发现TrkA mRNA原始模板浓度存在显著降低[(4.63± 2.15)×10-5 vs (22.32±13.31)×10-5],从而提示TrkA mRNA表达水平明显降低(P<0.05,图 2)。
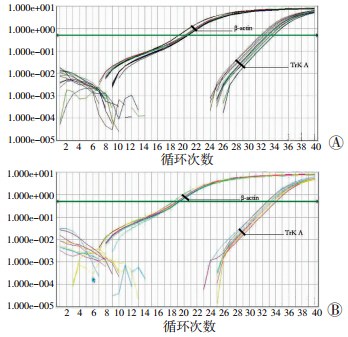 |
| A:假手术组;B:POL组 图 2 实时荧光定量PCR检测压力超负荷大鼠心肌 TrkA mRNA表达 |
由神经支配的靶组织细胞合成、分泌的NGF蛋白主要发挥维持神经生长、促神经修复、再生作用,并影响交感神经突触功能、促进再摄取[8, 9]。研究显示,心 血管病时心肌NGF及受体表达异常与心脏交感去神经或功能性交感去神经(functional sympathetic denervation)关系密切[2]。
研究观察到,压力负荷大鼠心脏交感神经密度增加,伴有交感神经再摄取功能减退,即功能性交感去神经(functional sympathetic denervation)[2, 3]。本实验大鼠POL 8周后心肌肥厚,心功能代偿,心脏交感神经NET mRNA 表达无明显变化,与文献[2]报道一致。可能说明,心脏NET蛋白表达/功能变化可能是导致交感神经再摄取异常的主要原因。分析原因:①NET mRNA转录后异常影响心肌组织NET蛋白表达。Backs等[10]报道,大鼠主动脉缩窄HF模型心肌NET蛋白减少,交感神经再摄取功能下降,而mRNA表达水平无变化,认为转录后异常可能是NET蛋白减少的原因;②NET内化(internalization)可导致再摄取功能减退。心脏交感神经NET膜表达(surface expression)是其发挥正常功能的条件。神经元蛋白激酶C(protein kinase C,PKC)激活,通过神经元细胞膜上的脂阀(lipid raft)介导NET内化[11]。推测POL后心脏交感神经突触前膜PKC激活可能对NET功能降低发挥重要影响。
本实验还观察到,长期POL诱导心肌NGF蛋白表达明显增加,但交感神经生长标志——心肌GAP-43 mRNA水平无明显变化。GAP-43与神经轴突生长密切相关,是神经重塑、再生的分子标志物[12],说明心肌NGF蛋白增加并未导致心脏交感神经生长。研究证明,NGF主要通过两种受体——高亲和力TrkA和低亲和力受体P75NTR介导生物学作用,以TrkA受体介导为主[13]。本实验大鼠POL后心肌TrkA mRNA表达明显降低,可能限制NGF促神经生长作用。然而,从另一角度看,TrkA受体表达降低可能限制NGF导致的交感神经过度生长,对降低交感神经再生相关的心律失常可能有利。研究表明,补充或过表达NGF蛋白与诱发心律失常关系密切[14]。
目前研究显示心脏POL大鼠心肌NGF蛋白水平变化并不完全一致[15, 16],我们观察到POL后8周心功能代偿,此时大鼠心肌NGF蛋白表达增加,可能与POL后不同时间阶段有关,应作进一步观察。有研究报告,随POL时间延长,大鼠心肌NGF蛋白从无明显变化到显著增高[16]。此外,POL后心脏交感神经密度与NET功能变化的关系还需进一步实验证明。
| [1] | Lymperopoulos A, Rengo G, Koch W J. Adrenergic nervous system in heart failure: pathophysiology and therapy[J]. Circ Res, 2013, 113(6): 739-753. |
| [2] | 李贺, 周欣, 王珂, 等. 心脏交感神经和心肌间质重塑的共同通路-蛋白激酶C途径[J]. 生命科学, 2011, 23(1): 57-62. |
| [3] | Kimura K, Kanazawa H, Ieda M, et al. Norepinephrine-induced nerve growth factor depletion causes cardiac sympathetic denervation in severe heart failure[J]. Auton Neurosci, 2010, 156(1/2): 27-35. |
| [4] | Kimura K, Ieda M, Kanazawa H, et al. Cardiac sympathetic rejuvenation: a link between nerve function and cardiac hypertrophy[J]. Circ Res, 2007, 100(12): 1755-1764. |
| [5] | Grossman W, Paulus W J. Myocardial stress and hypertrophy: a complex interface between biophysics and cardiac remodeling[J]. J Clin Invest, 2013, 123(9): 3701-3703. |
| [6] | He B, Ye F, Zhou X, et al. Exogenous nerve growth factor supplementation elevates myocardial immunoreactivity and attenuates cardiac remodeling in pressure-overload rats[J]. Acta Biochim Biophys Sin (Shanghai), 2012, 44(11): 931-938. |
| [7] | Li H, Ma X Q, Ye F, et al. Expressions of cardiac sympathetic norepinephrine transporter and beta1-adrenergic receptor decreased in aged rats[J]. J Zhejiang Univ Sci B, 2009, 10(3): 203-210. |
| [8] | 张玉波, 伍亚民, 杨恒文, 等. NGF促周围神经再生过程中对血管生成的影响[J]. 第三军医大学学报, 2005, 27(14): 1463-1466. |
| [9] | Skaper S D. The neurotrophin family of neurotrophic factors: an overview[J]. Methods Mol Biol, 2012, 846: 1-12. |
| [10] | Backs J, Haunstetter A, Gerber S H, et al. The neuronal norepinephrine transporter in experimental heart failure: evidence for a posttranscriptional downregulation[J]. J Mol Cell Cardiol, 2001, 33(3): 461-472. |
| [11] | Nelson T J, Sun M K, Hongpaisan J, et al. Insulin, PKC signaling pathways and synaptic remodeling during memory storage and neuronal repair[J]. Eur J Pharmacol, 2008, 585(1): 76-87. |
| [12] | Meiri K F, Pfenninger K H, Willard M B. Growth-associated protein, GAP-43, a polypeptide that is induced when neurons extend axons, is a component of growth cones and corresponds to pp46, a major polypeptide of a subcellular fraction enriched in growth cones[J]. Proc Natl Acad Sci USA, 1986, 83(10): 3537-3541. |
| [13] | Zhou S, Cao J M, Swissa M, et al. Low-affinity nerve growth factor receptor p75NTR immunoreactivity in the myocardium with sympathetic hyperinnervation[J]. J Cardiovasc Electrophysiol, 2004, 15(4): 430-437. |
| [14] | Feng N, Hoover D B, Paolocci N. Forever young? nerve growth factor, sympathetic fibers, and right ventricle pressure overload[J]. Circ Res, 2007, 100(12): 1670-1672. |
| [15] | Shyu K G, Liou J Y, Wang B W, et al. Carvedilol prevents cardiac hypertrophy and overexpression of hypoxia-inducible factor-1alpha and vascular endothelial growth factor in pressure-overloaded rat heart[J]. J Biomed Sci, 2005, 12(2): 409-420. |
| [16] | Nyquist-Battie C, Cochran P K, Evans V R, et al. Regulation of sympathetic presynaptic components in rat left ventricle during ligation of abdominal aorta[J]. Am J Physiol, 1996, 271(4 Pt 2): H1547-H1554. |



